Chrome is a metal that is characterized by good strength, pleasant appearance and high corrosion protection. In practice, products made from pure chromium are rarely used due to the high cost of the metal (difficult mining, difficult beneficiation). Therefore, this chemical element is usually applied in a thin layer to other metals, stone, plastic, wood and other materials. This allows you to improve the physicochemical and aesthetic properties of the workpiece for a relatively small amount. In metallurgy, the procedure of applying a thin layer of chromium is called chrome plating.
Various products can be coated with chrome - work tools, car wheels, equipment for ships, dishes, decorative items. Chrome plating can be performed in various ways, and today there are more than 10 such methods (although in practice 3-4 chrome plating technologies are used). But is chrome plating possible at home - or is it only carried out in factories and factories? What are the features of chrome plating of plastic? And what safety rules should you not forget about? These issues will be discussed in the article.
What is chrome plating?
Chrome plating of parts involves applying a special coating to a metal surface. There are two main methods:
- Saturation of surface layers by diffusion method. The maximum number of chromium atoms penetrates into the metal crystal lattice.
- Electrolytic method. It involves the cathodic deposition of chromium onto a steel structure under the influence of an electric current.
Chrome plating is carried out only on a clean, prepared surface. A feature of the process is the application of a uniform layer of a certain thickness to structures of various shapes. The coating can perform a decorative or protective function.
The thickness of the formed layer is from 0.075 to 0.25 mm. Hardness - 66–70 HRC. The surface has roughness and the thicker the applied coating, the more defects there are. Therefore, after chrome plating, polishing of the workpieces is required.
The technology involves the use of solutions:
- with chromic anhydride;
- with chromium sulfate or chloride.
The need for chrome plating
Metal chrome plating refers to the process of metallization with chromium to improve the surface properties and characteristics of elements. During chrome plating, various steel surfaces are diffusely saturated with chromium. Chromium treatment is also acceptable for ABC plastic, aluminum, brass, and silumin.
Chrome plating gives the parts a more beautiful appearance and improves their appearance. The chrome layer provides the original metallic color; car alloy wheels, headlight reflectors, motorcycle parts, souvenirs or home furnishings begin to look more aesthetically pleasing.
Other advantages of chrome plating:
- Protection. Applying a layer of chromium helps to increase the resistance of products to temperature changes, increases corrosion and erosion resistance, and reduces susceptibility to mechanical damage. The parts become super-hard (950 – 1100 units on the corresponding scale), therefore they react less to chemical damage and do not oxidize.
- Recovery. The service life of the base is significantly increased, large and small parts become very resistant to wear. At a low level of wear, chrome plating completely restores the product (for example, cracks up to 1 mm deep in shafts and bushings are closed).
- Reflective qualities. Some elements of the car are chromed to increase visibility in the dark. Reflection improves the decorative qualities of equipment.
- Purity. Chrome plating of products will protect them from dirt and dust, as it prevents the adhesion of various contaminants.
Compared to nickel plating, chrome plating has fewer disadvantages: the cost of services is lower, the coating will be harder and more durable. The use of nickel benefits only in terms of decorative qualities, since the surface becomes even more aesthetically pleasing.
Where is it used (purpose of chrome coating)
- In industry, many products are subject to wear and tear, such as molds or piston cylinders. They are made of carbon steel or stainless steel. Without coating, such molds quickly wear out and must be replaced. When they are coated with hard chrome, their service life increases by more than 3 times.
- Chrome metal is a food grade coating. This means that it does not react with food and does not cause allergies upon contact with the skin and mucous membranes of people and animals. Therefore, it is constantly used to cover surgical instruments (scalpels) and mechanical parts that come into contact with food.
- Chromium is stable in vacuum. They cover parts of spaceships.
- Chrome is heat-resistant and is used for products that constantly operate at high temperatures.
There are many other purposes for this coating.
Features of chrome plating
Although chrome plating can instantly change the appearance of a motorcycle or car, this procedure is quite complex and dangerous.
This is due to the use of a special galvanic bath and serious reagents that can be harmful to health if the instructions are not followed. All chemical and physical transformations must occur under the strict supervision of a specialist, even if the procedure is carried out at home. Therefore, it is important to familiarize yourself with the entire theoretical basis before starting. Since chrome plating metal at home became a trend, a lot of different methods have appeared. Chromium deposition is a physical and chemical process that occurs after the part is treated in a galvanic bath. A thin layer of metallic chrome is applied to the product, which hardens almost instantly. As a result, it is easy to obtain a body part with a shiny surface and resistance to external damage. Chrome plating significantly enhances the physical, chemical and decorative characteristics, so it is often used on the lower parts of the vehicle that are exposed to the external environment.
Chrome plating technologies at home
You can order the services of experienced companies that carry out the procedure, or do it yourself, but in any case, the meaning of the operation is to clean the surface followed by treatment with current and reagents. The chemical laws of catalysis and oxidation are at work here, so you need to get a little familiar with this science. Below we will tell you more about the processes.
Types of metallization
Galvanic chrome plating
Chromium plating is carried out by deposition on the surface of parts of a layer of metal from electrolytes containing ions of this metal. Two anodes are mounted in a special container and the electrolyte is poured. The chrome-plated product is connected to the “minus”, and the anodes to the “plus”. Next, the circuit is closed and the metal that is part of the electrolyte is deposited on the part. Electroplating chrome plating is a very good metallization option for metals. As a result of this process, a mirror-like, smooth surface is obtained. It has high anti-corrosion properties, wear resistance, and can also withstand high temperatures. Depending on the use of different electrolytes, the process may be called galvanic gold plating, silver plating, or copper plating.
It should be noted that applying metal by electroplating is a toxic and dangerous process associated with the use of chemically active substances that are harmful to health. It is necessary to take this into account and ensure good ventilation and use personal protective equipment.
Vacuum metallization
Vacuum deposition is carried out in special vacuum chambers. Under the influence of an electromagnetic field, a thin layer of metal is deposited. Next, the surface is filled with varnish.
This process is used as a decorative type of chrome plating.
The advantage is low cost and visual similarity of the result to galvanic chrome. The disadvantage is that a product chrome-plated in this way does not have high wear resistance. It also requires expensive equipment that requires a lot of electricity and materials to operate.
Decorative silver plating
This process is often mistakenly called chrome plating. It is not chrome, but silver that is sprayed onto the part. It’s just that the word “chrome plating” is on everyone’s lips. We will consider this metallization method in more detail, since it is the most acceptable from the point of view of the equipment and chemical reagents used. Also, the application method itself is not complicated.
The full name of the process is chemical silver plating by sputtering. A special composition is sprayed onto the surface. The method of watering and dipping is also used.
This is how surfaces are metallized mainly with nickel and copper.
When silvering surfaces, the spraying method is used. Silver gives a wonderful shine. You can silver plate any hard surface.
How to silver plate parts?
The composition should be sprayed onto a glossy surface. A thin layer is applied that follows all the irregularities. Thus, to obtain a “mirror”, the smoothness of the product is important.
Stages of silvering parts:
- Preparation. If necessary, you need to putty and sand the surface. Next, prime, prepare the soil and degrease. When using a special adhesive primer for metallization, you can avoid subsequent varnishing before the silvering process, and immediately apply silver to the primer. It forms a glossy surface on the surface of the product. The primer for metallization spreads well without forming shagreen.
- Varnishing. After the varnish has dried, the part is ready for silvering.
- A special solution (sensitizer/activator) is sprayed onto the surface with a spray gun, washed with another spray gun, silvered with a third, then washed again, applied remover, washed again. Finally, blow off the remaining water with a blow gun.
- Next we coat it with protective varnish. Without it, the coating is easily damaged by light physical impact.
By adding various dyes to the protective varnish, you can get any color.
Silver plating tool
Air guns for silver plating
Silvering can be done with a “single-barrel” gun, into which two chemical hoses and one air hose are inserted. You can also silver plate “double-barreled” pistols. For silvering, it is necessary that two chemical solutions (silvering and reducing) are mixed with each other and then sprayed onto the surface. In a “single-barrel” pistol, mixing is carried out inside the pistol, and in a “double-barrel” pistol, mixing is carried out outside. You can work with any pistol, using a good, high-quality composition. If there are doubts about the quality of the chemical composition, then it is better to use a “double-barreled” pistol.
Attention: chemicals used in the silver plating process contain toxic substances!
Always use special protective equipment!
Yellowness with silvering
Appears when silver is coated with varnish. You can minimize this effect by adding blue-violet toner to the polish.
Protective varnish
The varnish shrinks in size as it dries. It seems to shrink and thus can slide off the surface of the silver-plated part. This happens because the varnish sprayed onto a mirror-like smooth surface has nothing to catch on. The varnish still adheres to the surface of the silver due to its thickness and strength. If such a product is not actively used, the protective varnish will not peel off. If there is a mechanical impact on the coating, the varnish may not “resist”.
To improve adhesion, special plasticizers are mixed into the protective varnish. It becomes more elastic and soft, shrinks less when drying and holds better. But this is not a panacea and does not greatly improve the adhesion of the protective varnish to the surface.
A special coating is also used, which is sprayed after applying silver. It is applied in two layers. The first layer is lightly sprayed, and the second is poured onto the surface. Next, this coating is dried and filled with protective varnish. Then it is dried until it is “touch-free” and again filled with protective varnish. This coating is durable.
There are powder, dry varnishes that have good adhesion to metals. Powder varnishes are activated at a temperature of about 200 degrees Celsius. Unfortunately, at this temperature the silver plating becomes dull. Thus, powder varnishes are not suitable for protecting silver-plated parts.
Advantages of silver plating technology:
- Simple technology that does not require complex equipment. Wide selection of equipment and materials. Low cost of coating.
- Silvering does not require large areas. A small room with a hood is enough.
- Silver plating provides high reflectivity and, due to its decorative properties, can be an alternative metallization method instead of the more complex methods of galvanic chromium plating and vacuum metallization.
- You can silver plate any hard surface, not just metal (in the case of electroplating).
- Products can be coated not only with chrome. By adding special dyes to the protective varnish, you can change the shade of the final decorative coating.
The main disadvantage of decorative silvering is the poor protective properties of the coating. If you can properly protect the silver-plated surface, this drawback will be eliminated. That is, it is important to properly coat with protective varnish, as described above.
It is also worth mentioning paints with a mirror effect.
Chrome plating methods
Galvanization technology consists of applying a surface metal layer to a part for a specific purpose - decorative or protective through the use of an electrolyte.
Consequently, electroplating - chrome plating at home suggests that a layer of chromium from an electrolyte of a certain composition will be deposited on the surface being treated when exposed to electric current. This process can be carried out in different ways.
- Carrying out chrome plating using a bath filled with a solution. The technology is accessible for DIY, but is more often used when working with small parts.
- Spraying the coating using a galvanic brush. This technique does not limit the dimensions of the workpiece; it is also optimal for non-removable elements. During the work process, the master has the opportunity to control the thickness of the applied layer and visually assess the quality of the spraying. But this process is more labor-intensive, as it may require up to 20 movements in one place.
Galvanic chrome plating method
Galvanic chrome plating of parts is the most popular method, because all the steps can be done with your own hands. Electroplating involves placing parts in a special solution with a certain composition, from where, under the influence of waves (solitons) of electric current, chromium atoms will be deposited on the surface. Having the right set of tools for chrome plating, you can create a high-quality coating yourself by galvanizing.
Electrolytic chrome plating method
One of the types of galvanizing. When electrolysis is used, tri- or hexavalent chromium gives the product the desired “metallic” appearance. When using a trivalent element, the main substance of the solution is chromic anhydride. The use of hexavalent chromium differs from the previous method by the presence of chromium sulfate in the solution.
When carrying out electrolytic chrome plating of disks or other parts, it is important to strictly observe the proportions of the components. Otherwise, the protective layer will quickly peel off or there will be stains, uneven dullness and insufficient gloss.
Diffusion chromium plating method
Chromium coating is done using a galvanic brush. At home, this method is more preferable, because the master does not need to use a bath. It is especially recommended to perform the technique for parts made of aluminum, carbon steel, and alloys with silicon.
Chemical metallization of surfaces and parts
In the process of this work, chemical reagents, a compressor and a spray gun are used. Almost the same operations are performed as when painting surfaces with acrylic varnish or enamel. When chrome plating in this way, not a protective polymer film is applied to parts and structures, but a mirror-like thin layer of metal. Its thickness is in the range of 0.075-0.25 millimeters. The chemical and physical characteristics of such a coating are comparable to those obtained by vacuum deposition.
Catalytic chrome plating method
A subtype of chemical chrome plating of ferrous or non-ferrous metals, which involves applying a liquid without acids to the part. The technology is safe for humans and helps create original, unusual effects.
Catalytic chrome plating can be used for conventional and flexible products (with electrolysis, the latter is impossible; the coating will peel off).
Typically, silver is used as a reagent in an alkaline ammonia solution, and formaldehyde or hydrazine is used as a reducing agent. The use of silver makes the piece look milky with a mirror surface.
Chrome plating using chemical metallization
The chemical metallization method is most suitable for chrome plating parts at home. There are also special reagents here, but no other complex equipment is required except a spray gun and a compressor.
In many ways, the chrome plating process is reminiscent of conventional painting of products with enamel or acrylic colored varnish, only the result is different. Instead of a polymer protective film, a thin (0.075 - 0.0) is formed on the surface.
25 mm) a metal layer with a mirror shine, not inferior in physical and chemical characteristics to vacuum deposition.
There are two methods of chemical metallization:
- recovery of chromium from salts during chemical reactions;
- coating with specially selected chemicals, as a result of the interaction of which a durable monomolecular layer of silver or other metal is deposited on the surface.
To work with chromium salts, you will need sodium hyposophyte, chromyl fluoride, chromium phosphate, chromium chloride or acetate, caustic soda, acetic acid and other chemicals.
Most of them are unsafe for health, some are very poisonous. If you intend to chrome parts using this method, you need to repeat the chemistry course.
Even with carefully written instructions, it is difficult to achieve the desired result.
Vacuum chrome plating
The technology belongs to chemical metallization and has another name - the PVD process. Gives condensation of chromium vapor on the surface of the part after placing it in a special vacuum chamber. In this installation, under negative pressure, chromium is heated to the evaporation temperature, then settling like fog on the product.
Calculation of pressure and chrome plating period will depend on the degree of wear of the part and the type of material. After vacuum chrome plating, the thickness of the metal layer is minimal, so the part on top is coated with special spray paint or varnished.
Thermochemical chromium plating
Powdered products consisting of fireclay and ferrochrome are used. The technique is similar to that for chemical chrome plating, only the product will be subject to heating during the process.
Spray processing
Sputtering of elements with chromium (catalytic metal working) is performed using the “silver mirror” reaction, in which complex silver layers located in alkaline ammonia solutions act as reagents. The function of a reducing agent is assigned to a formaldehyde or hydrazine solution, or inverted sugar syrup. When silver and a reducing agent are simultaneously sprayed, the steel product acquires a boiling white surface with a mirror shine. Such products are characterized by excellent reflective characteristics. The next stage involves applying a varnish coating to the product to protect it from scratches with the addition of a light-fast coloring toner. To obtain the latter, three pigments are mixed - black, blue and violet in a ratio of 1: 1: 3. Processing using a “silver mirror” is divided into a number of successive steps :
- Analysis and preparation. First, the surface of the product is thoroughly cleaned by rinsing with a specialized composition. To increase adhesion, the surface is sanded with sandpaper with an abrasiveness level of P500−600.
- Application of a glossy base. A glossy black substance is applied to the prepared material, which eliminates the risk of yellowness in the final mirror coating. Then the varnish coating is dried at room temperature from 20°C to 25°C, without the use of specialized equipment, and drying lasts 8 hours. If drying is carried out at a temperature of 60°C, the process duration is reduced to 45 minutes.
- Drying.
- Etching the surface of the product to increase its adhesion to silver, followed by cleaning with distilled water.
- Sensitization or specialized surface treatment using an activator. This allows you to protect the surface with a special film.
- Metallization using silver.
- Application of a protective varnish coating to the product, which reliably protects it from tarnishing and loss of original qualities during long-term use, mechanical stress, or due to the destructive influence of aggressive environments.
Work order
For most chrome plating methods, the product will be coated in the following order:
- Cleaning from heavy contaminants and preparation. Removing excess oil, old coating, disconnecting moving parts of mechanisms, fastening products to equipment for coating.
- Degreasing. Removing the smallest parts of fat on the surface using solutions. There are several options: Chemical degreasing (washing powder), electrochemical, ultrasonic, etc.
- Pickling (for steel products). Removing rust and scale.
- Coating.
- Drying.
- Quality control.
Process description
Our contemporaries are not much different from representatives of past eras in terms of their love for attractive, sparkling things. Originally designed car body parts, sparkling chrome plumbing in the kitchen and bathroom, unusual figurines and beautiful façade coverings of buildings - all this is extremely popular today, which is why the need for the services of craftsmen who know chrome plating technology is constantly increasing. Today, 3 methods are widely used metallization of workpieces:
- galvanizing;
- chrome plating;
- aluminizing (aluminizing).
Zinc coating makes steel and other metals resistant to corrosion, which can greatly increase the life of products coated with zinc. Aluminum also increases resistance to corrosion, for this reason it is often coated with devices designed to operate in a temperature range of up to 900 ° C. Such equipment includes equipment for oil production and pumping liquefied gas, units and parts of furnaces, and much more. Applying a thin layer of chrome allows you to create fashionable decorative coatings and make products more aesthetically pleasing, skillfully hiding factory defects. Chrome plating also improves performance, including:
- Increases resistance to corrosion;
- Makes metal harder;
- Better protects the surface from erosion;
- Makes the product heat-resistant;
- Increases wear resistance;
- Improves the appearance of the product;
- Makes it possible to create high-quality coatings with established characteristics.
Galvanization
The galvanic method is the coating of cast iron, steel, brass or copper structures with a layer of chromium. But not only metal products can be chrome-plated by galvanization. This method can also be used for chrome plating of plastic and wooden products. But in these cases the process will be expensive and technologically complex. To firmly hold the chrome coating on the surface of parts, even metal products require another preliminary coating. For this purpose, nickel, brass or copper are used.
Galvanization requires the creation of a galvanizing plant. In addition, you need a DC source and a set of reagents. This set consists of chromium anhydride, sulfuric acid, soda ash and sodium hydroxide.
It should be noted that when working with this method, it is required that there are no changes in current strength. You also need to constantly monitor the level of salt concentration in the electrolyte and strictly observe the temperature regime for quite a long period (from 5 to 8 hours). Fulfilling all of the above conditions in home workshops is not an easy task. It is for this reason that we will not describe the galvanization process in detail in this review.
How to chrome parts?
Do-it-yourself chrome plating of structures at home must be done in a spacious non-residential area. You need to prepare tools, thick clothing, glasses, and a respirator.
To prepare the solution, you need to use non-metallic containers. This is due to the need to use an oxidizing solution.
Thin sheet lead or tin alloy should be used as the cathode. The electrolysis bath can be plastic, cylindrical or rectangular. If the solution has been prepared in more than the required volume, it can be stored in an airtight container with a lid.
To prepare the electrolyte, only pure substances should be used. Chromic anhydride is not commercially available.
Step-by-step chrome plating of car parts:
- Clean the part from dirt and polish it.
- When processing steel, surface activation is carried out. It involves immersing the workpiece in hydrochloric acid for 5–20 minutes. The duration depends on the size and complexity of the surface.
- Residues of acid are washed off the part.
- After drying, the structure is immersed in a bath of electrolyte solution.
- An anode lead plate is installed inside the bath, and the positive contact from the current source is connected to it. The negative wire is connected to the part.
- The power is turned on for 20-40 minutes. After time has passed, the part is removed.
- After 3 hours, the chrome surface is polished to a shine.
To obtain a high-quality coating, you need to ensure:
- stable electrical voltage;
- compliance with electrolyte proportions;
- preparation of the part in accordance with technology requirements;
- temperature control and compliance;
- keeping the electrolyte under current for a certain time (from three hours).
Degreasing
You can limit yourself to gasoline or solvent, but if greasy stains still remain, it is most effective to soak the item for 20-60 minutes. Liquid composition:
- 1 liter of water heated to 80-90 degrees.
- Sodium hydroxide – 150 g.
- Liquid glass glue – 5 g.
- Sodium carbonate (technical soda) – 50 g.
Preparation of the workplace
Metallization with chromium is a chemical process accompanied by the release of toxic (carcinogenic) substances that are harmful to human health and the natural environment. Therefore, for electroplating at home, a non-residential, impeccably ventilated room is selected. The best choice is a garage or a detached workshop with effective forced ventilation (exhaust). Consideration should be given to waste disposal.
Chromium electrolyte releases volatile compounds that can come into contact with and destroy any organic matter. Vapors are hazardous to the skin and mucous membranes. To protect against fumes, use goggles and a respirator mask.
Chrome plating at home is carried out in overalls, boots and an apron. Protect your hands with thick latex or rubber gloves. Before work, it is recommended to lubricate the nasal cavity with an ointment consisting of Vaseline and lanolin (in a ratio of 2 to 1).
Selecting a room
Chrome plating should be carried out in a well-ventilated non-residential area. The ideal option is a car garage. In summer you can work right outside under a tarpaulin canopy. Such measures are mandatory, otherwise the health of the performer may be in danger due to toxic and poisonous fumes of the substance.
Surface preparation
This important stage should be given special attention. After all, you can get a high-quality coating only on perfectly cleaned and grease-free surfaces. Therefore, before chrome-plating metal, wood or plastic at home, they must be thoroughly cleaned.
The parts being processed should be free of dust, dirt, and the slightest traces of rust. Old coating and paint should also be completely removed.
After cleaning, uneven surfaces are thoroughly sanded. There should be no chips or scratches left. The next stage is polishing with sandpaper and special pastes.
Then the part to be chrome-plated is degreased in a solution heated almost to boiling (90°C). Products made of non-ferrous metals can be treated with a mixture of soap without cosmetic additives and sodium phosphate. Steel or cast iron is immersed in a solution of liquid glass, caustic soda, sodium carbonate and sodium phosphate. Sulfuric acid is used to remove the oxide film.
Chromium plating factors
The constant temperature of the electrolyte must be no less than 50 and no more than 55 degrees. First place the item in the container, and only then apply electricity so that the metal heats up to the temperature of the water.
Required Equipment
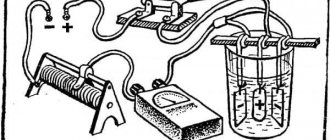
Electroplating (chrome plating) at home is possible if you have the following type of equipment:
- power supply: it should show 1A at the output and be equipped with a voltage regulator; for small volumes of work, a current rectifier is sufficient; the wiring cross-section depends on the size of the workpiece (minimum 6.25 mm);
- wires: the positive one will be immersed in the electrolyte, the negative one, with an alligator holder, will be located at the end towards the workpiece;
- anodes made of tin, lead or antimony alloys;
- containers of suitable size made of chemically resistant, non-conductive material; the ideal option is a plastic bath; for chrome plating of small parts, a glass jar is sufficient;
- a wooden box with thermal insulation made of glass or mineral wool in which the container will be placed; Ordinary sand can also be used as insulation;
- sealed lid: it can be made from a piece of plywood or wooden boards;
- heating element, the power of which is sufficient to heat the liquid in the selected container to a temperature of 60-80°C;
- contact thermometer or thermostat;
- a hollow mold for pouring electrolyte with a tap or brush at the end; to make it, a bundle of copper wire is used, secured and tied with lead wire.
Means of protection
It is also necessary to acquire protective equipment - thick rubber gloves and a high-quality respirator. Clothes can be covered with an apron made of rubberized material.
Power supply
For electroplating in a home laboratory, a grounded DC source with an adjustable voltage of 1.5-12 V, with a maximum current of 20 A, is suitable (it is convenient to use a rheostat to adjust the output power).
The cross-section of the connecting wires is selected taking into account the maximum load (current). For chrome plating of small parts, wires with a cross section of 2.5 mm are used.
Chrome plating with a brush
The main element of equipment necessary for chrome plating plastic at home using a special brush is the brush itself, with which the reagent is applied to the surface of the workpiece.
Scheme of a brush for chrome plating
You can do it yourself. To do this, you need to take a tube made of organic glass, hollow inside, and attach bristles made of electrically conductive material to one end of it. A bundle of thin bare copper wire is best suited for this purpose. The bristles of the brush should be wrapped with thin lead wire.
To apply chrome coating to plastic, the part itself and the brush must be connected to a power source, such a source can be a transformer or a car battery. Depending on the choice of power source, the connection diagram will be different.
If a transformer is used, a diode is connected to the hand: the anode is connected to the step-down winding of the transformer, and the cathode is connected to the workpiece using an alligator clip. If the power source is a battery, no diode is used.
After connecting to a power source, electrolyte is applied to the part using a brush, which is first poured into the hollow handle of the brush; it is important to monitor the level of the electrolyte itself. The solution is applied with smooth movements from side to side in even layers.
In order for the coating to last as long as possible, experts recommend applying the solution in several layers, the number of passes over each area should be in the range from 25 to 35 times.
Preparing the container for chrome plating
In any case, to perform the procedure, you will need a conditional bath to keep the part in the working solution. Depending on the size of the part, a 3-liter jar, basin, tank or classic bath can be used. The only limiting parameter of choice is the material of manufacture - glass or metal that does not enter into chemical reactions with the electrolyte. Next, you should start arranging the selected container.
First, it must be installed on a flat surface and locked in a stable position so that the chrome plated metal receives an even coating. Secondly, in cold weather the container will need thermal insulation, which can be fiberglass, mineral wool or bulk material (sand, expanded clay).
At the same stage, devices or structures are thought out for conveniently holding the workpiece and functional devices that will be used at different stages of the operation.
Preparation of electrolyte solution
To carry out chrome plating of parts at home, you need to prepare a special solution consisting of chromium anhydride (250 g per liter of distilled water) and sulfuric acid (2.5 g per liter of water).
First you need to fill half the container with heated water (about sixty degrees Celsius). Add the required amount (based on the total displacement) of chromium anhydride, stir until completely dissolved, and add water to obtain the required volume. Then add sulfuric acid, stirring the liquid.
The resulting solution must be worked for three and a half hours, passing current energy through it (about 6 A per 1 liter). When the electrolyte turns dark brown, it will need to stand for at least a day.
Preparing the workpiece
The quality and durability of the applied chrome layer depends on the preparation of the part to be painted. The chrome surface must ideally satisfy all required cleaning parameters. This can be done while the finished solution is settling after “working through” with electric current.
Cleaning and degreasing
The metal part must be completely cleaned of any debris, paintwork, primer, rust, etc. This stage of preparation should be treated with special attention, because the quality of chrome plating depends on it.
Even if the part has been painted frequently, sandpaper or a special grinding machine will help to cope with this. When using abrasive attachments or hard disks similar to sandpaper, cleaning the surface of a metal product is not difficult. A sanding machine will even help smooth out all scratches and chips, making the surface perfectly smooth. After the surface is completely cleaned of dirt and paint, you should proceed to degreasing. The quality of the application of the chromium layer also depends on the quality of this procedure.
Degreasing is the preparation of a special solution that includes the following components:
- sodium hydroxide - 150 g/l;
- soda ash - 50 g/l;
- silicate glue - 5 g/l.
After mixing the solution to degrease the parts, it is heated to a temperature of 80-90 degrees Celsius. Products are kept in it for 20 minutes, but if the surface has a complex topography or is heavily soiled, the time increases to 1 hour.
How to prepare electrolyte?
To chrome plating parts at home, you need an electrolyte. Proportions of ingredients for preparing the solution:
- anhydride in solution 150–250 g/l;
- sulfuric acid from 1.5–2.5 g per liter of solution.
Chromium anhydride is poisonous and its use is deadly.
Electrolyte preparation:
- The glass vessel is filled 50% with water heated to +600C.
- Anhydride is added according to the amount of water poured. The mixture is stirred until completely dissolved.
- Add water until the vessel is full.
- Acid is poured in proportion and the liquid is thoroughly mixed.
During electrolytic reduction, the cathode is attached to the workpiece, and the anode is immersed in the prepared solution.
The solution remaining after chrome plating must be disposed of. It is carcinogenic and can cause skin diseases and tumors.
Compound
Dissolve 250 g of CrO3 - chromic anhydride in one liter of clean water (preferably bottled distilled or regular tap water, but after filtering and freezing). Then you should add 2.5 grams of H2SO4 - this is sulfuric acid, it must be concentrated (calculation for density 1.84). You can buy all this in specialized stores.
Cooking method
Instructions for making electrolyte:
- Heat the water to 60 degrees, fill 1/2 of the container with it.
- Add CrO3, dilute completely.
- Add the remaining heated liquid.
- Add sulfuric acid and stir.
- Pass current for 3.5 hours. Its strength is calculated from the formula - 6.5 amperes per liter. After this, the electrolyte will turn brown.
- Place the tank in a cool, dark place for a day.
Part preparation
Produced in two stages. First, take the pre-prepared solution, reheat it to 60 degrees, and then leave for three hours. During this period, it is just possible to clean and degrease.
Diffuse galvanic treatment method
Apply the method of heat treatment of steel using chrome plating, which has a positive effect on the operational properties of the surface, giving the material strength, hardness, toughness, wear resistance, elasticity, heat and corrosion resistance. Subject to a certain temperature regime, the surface of a particular workpiece is susceptible to the action of reagents, and through diffusion, the surface layer is saturated with chromium. Diffusion treatment is indispensable when applying silicon, carbon, nitrogen and aluminum to the surface layer.
Thermal chrome plating using powders involves the use of mixtures that consist of ferrochrome and fireclay. Such a composition is usually called solyanka acid. Another type of diffusion treatment is the condensation of chromium vapor.
Chrome plating stage
Do-it-yourself chemical metallization at home begins with heating the electrolyte in a jar to 52±2°, followed by placing a part into it, to which the cathode is pre-attached. The current is not applied immediately, since it is necessary for the object to be decorated to warm up to the temperature of the electrolyte.
After voltage is applied to the system, the part is in the electrolyte for at least 20 minutes. The optimal current density is 50 – 55 A/dm2. With experience, the home craftsman can easily determine whether it is necessary to increase the time depending on the characteristics of the part, since in some cases chrome plating can last two to three hours.
After the process is completed, the item is taken out, washed and placed in a drying cabinet for 3 hours.
When carrying out chrome plating operations, it is necessary to take into account that such a coating can be applied if the parts are copper, nickel or brass. If there is a need to perform a similar action with steel objects, then you will need to first protect them with layers of appropriate metals.
Plastic products can be chromed if they are treated with graphite powder or graphite-containing varnish at the preparation stage. Then, according to the electroplating technique at a current density of ≈ 0.7 A/dm2, a thin copper layer is applied using an electrolyte from the following components (g/l of water):
- copper sulfate – 35;
- concentrated sulfuric acid – 150;
- ethyl alcohol – 10.
After washing and drying, the part can be chrome plated.
Processing conditions
During the chrome plating process, regardless of the processing method used, the release of harmful fumes is inevitable, so you should immediately abandon residential premises. The optimal place is a garage, utility room or other technical room. But that's not all. It is necessary to consider stable ventilation with efficient exhaust.
You cannot rely on natural weathering, since harmful substances can have an impact already during the work. How to chrome metal at home without causing harm to health? Even if ventilation is available, personal protective equipment should be prepared.
The required kit includes construction glasses, a respirator, an apron and rubber-coated gloves.
What to consider
- First, effective ventilation. Moreover, it should not be natural, but forced (exhaust).
- Secondly, a respirator, special glasses, rubberized gloves and an apron are a must.
- Thirdly, how to dispose of “production waste”?
Room
If you want to chrome-plate the bumper and wheel rims, then the balcony is clearly not enough. You will need a separate, spacious room.
Preparatory activities
Firstly, in order to polish metal well, you need to have certain skills.
Secondly, how many can boast of knowledge of chemistry, in particular, the specifics of the electrolysis process? Who can choose the correct proportion of all ingredients? But accuracy is the key to quality.
Thirdly, where can I get the necessary materials? You can still buy acid, but what about anhydride? This substance is sold only to legal entities, and you won’t be able to go in and buy it “just like that”, like “green stuff” or a loaf of bread. Therefore, you will have to search through your friends. It would be good if there were such people. By the way, H2SO4 must be PURE, and not the kind that is sold for batteries.
Fourthly, will the “amateur” master be able to withstand the required “current” mode while preparing the solution?
If at least one of the preparation points is not completed with proper accuracy, everything else is “monkey’s” work.
Preparation for the procedure
The first step is to prepare protective equipment. Nessesary to use:
- protective glasses;
- respirator;
- closed clothes;
- gloves.
Important! The chemical chrome plating procedure must be performed in a ventilated room. The best choice would be a room with an installed hood or good ventilation.
Method of chrome plating plastic at home
To ensure chrome plating of plastic at home, it is advisable to make an electroplating brush (the method is also applicable for metal products):
- Bristles (suitable from a paint brush) with a diameter of 20-25 mm are tightly wrapped with lead wire. It is fixed at the end of a cylindrical vessel, which is filled with electrolyte. It is convenient to use a container made of plexiglass (control the solution level). A diode is attached to the other end.
- The circuit uses a step-down transformer (12 V, 0.8-1 A). The negative side of the transformer is attached to a chrome-plated object (with an alligator clip). The plus goes to the anode of the diode, the cathode of the diode is connected to the bristle winding.
- A layer of liquid is applied to the surface to be treated with smooth, uniform movements; each section is passed with a brush at least 20 times, without lifting it from the surface.
- Once electroplating is complete, the item is washed and dried; dirt is removed with a compressor.
Health Hazard
Yes, chrome-plated metal is a corrosion-resistant material, but such a useful coating must be applied with the greatest care.
Because one of the two main components of the electrolyte, namely the anhydride (CrO3), is very toxic. Both in the form of crystals and dissolved in water and forming acids, it is a source of carcinogens. Cr salts and oxides are low-volatile, but this should not be reassuring, since as a result of heating (under the influence of an electrolyte) they can evaporate, mix with water vapor and then settle on the skin.
This is why it is so important to work in goggles, a respirator, gloves and overalls - so that harmful substances get on the fabric. Remember, if such a harmful substance is absorbed through the pores, enters the body through mucous membranes or in some other way, this is fraught with the development of serious diseases, including tumors. Therefore, it is simply necessary to strictly follow safety precautions.
We examined step by step how chrome plating of parts occurs, how to prepare for it, and what you need to remember when carrying out the process. Now that you understand all the risks of chemical deposition at home, we urge you to approach the procedure with the utmost responsibility and caution. can help you - we sell bandsaw machines, to clarify the information you are interested in, contact our managers at the contact numbers listed on the page.
Main defects and removal of low-quality chrome plating
Receiving a defective coating should not frighten a novice electroplater. A poor-quality layer of chromium can be removed in a solution of hydrochloric acid (100-200 g/l). After this, the parts are washed in water, and the chrome plating process can be repeated.
Most often there are several main defects:
- Peeling of the chrome film. The main reason is poor adhesion due to insufficient degreasing. After removing the coating, the surface is cleaned and reactivated again.
- Chromium growths (dendrites) on sharp edges and corners. This defect indicates a high current density on sharp edges. If possible, it is better to round the edges or install screens in problem areas.
- Matte finish. To achieve shine, it is necessary to increase the temperature of the solution, reduce the current, or add chromic anhydride.
Uneven surface shine
- High current strength.
- Electrolyte temperature is lower than recommended.
There is no "shine"
- Lack or excess of CrO3 in solution.
- The current rating is too high.
- The proportion of acid is less than required.
Brown spots on chrome plating
- Excess chromium.
- Lack of acid.
There are small shells on the coating
- Poor quality machining of the workpiece.
- Hydrogen is not removed from the surface during the reaction. In this case, you should change the method of “hanging” the sample and the drying method.
“Softened” coating
- Increased electrolyte temperature.
- Reduced current.
Chromium plating peeling
- Instability of supply voltage.
- Poor degreasing.
- The electrolyte has cooled down during processing for too long.
If someone thought that chrome plating is, in general, a simple thing, then they will have to be somewhat disappointed. Even if we are talking about a small “thing,” there are enough nuances. But it is especially worth warning about the pitfalls of those who are thinking of putting this business on stream at home.
Sources
- https://ometalledo.ru/kak-xromirovat-metall-v-domashnix-usloviyax.html
- https://pechistroy.ru/stroymaterialy/hromirovaniya-metallicheskih-izdeliy-v-domashnih-usloviyah.html
- https://obrabotkametalla.info/mexanizm/xromirovanie-detalej-v-domashnix-usloviyax
- https://www.rocta.ru/info/hromirovanie-detalej-svoimi-rukami-v-domashnih-usloviyah-kak-ehto-opisanie-tekhnologii/
- https://plavitmetall.ru/obrabotka/xromirovanie-v-domashnix-usloviyax.html
- https://pressadv.ru/samodelkinu/hromirovanie-detalej-v-domashnih-usloviyah.html
- https://fizmatlit.com/kak-hromirovat-metall-v-domashnih-usloviyah/
- https://boldproject.ru/raznoe/hromirovaniye-v-domashnih-usloviyah.html
- https://metalloy.ru/obrabotka/zashhita/kak-hromirovat-metall
- https://metmastanki.ru/hromirovanie-detaley-v-domashnih-usloviyah
- https://tokar.guru/metally/hromirovanie-detaley-v-domashnih-usloviyah-svoimi-rukami.html
- https://kraskaok.ru/hromiruem-detali-v-domashnih-usloviyah-tehnologiya-i-neobhodimoe-oborudovanie/
- https://kraskaved.com/kraski/rabota/smeshivanie-i-kolor/xromirovanie-svoimi-rukami.html
- https://ismith.ru/metalworking/xromirovanie-v-domashnix-usloviyax/
- https://unit-car.com/tuning/122-hromirovanie-detaley-v-domashnih-usloviyah.html