Protecting metal from the occurrence and development of corrosion is a very pressing issue, the solution of which can significantly extend the service life of metal products, as well as make their operation more reliable. The most common method to provide such protection is galvanizing, which involves applying a coating to the surface of the metal, the chemical composition of which can contain up to 95% zinc. Galvanizing of metal can be performed using various technologies, each of which is used in certain situations and has both advantages and disadvantages.
Galvanizing is the most widespread among other anodic protective coatings of metals.
Why is a layer of zinc applied to steel?
It is well known that products made of steel are very susceptible to corrosion processes, especially when used in conditions of high humidity. Meanwhile, if you galvanize a steel part, you can provide it with reliable protection against corrosion. This is explained by the fact that the zinc coating forms a galvanic couple with the base metal, in which zinc has a greater degree of electronegative charge than steel.
In such a galvanic couple, when its components are exposed to aggressive environmental factors, it is the zinc that is exposed to corrosion, and chemical reactions of steel are practically eliminated. Thus, corrosion protection of the steel will be ensured until the zinc coating is completely destroyed. At the same time, in those areas of the steel product where the zinc coating is destroyed for some reason, zinc hydroxide is formed under the influence of oxygen and moisture, which also has good protective properties.
Advantages of applying zinc before painting
Galvanizing steel products allows them to provide not only barrier, but also electrochemical protection. Galvanizing of metal can be carried out using different technologies, for the implementation of which various equipment is used. Using certain types of such technologies, you can perform galvanizing at home and still achieve excellent results.
Galvanizing a small part
The car part to be processed is prepared accordingly. Traces of corrosion and old paintwork are removed. When cleaning, do not use aggressive paint removers. If you still cannot do without them, then the part should be treated with an aqueous solution of soda.
Next, the required amount of zinc-containing solution and the appropriate container are prepared. The negative terminal of the battery is connected to the part, and it is immersed in the solution. The capacitance itself is connected to the positive terminal and voltage is applied. For successful galvanizing of a small part, a voltage of 12 V and a current of about 1 A will be sufficient. The result of the treatment should be a part with a uniform gray coating. Next, the element is removed and thoroughly rinsed in an aqueous soda solution.
Galvanizing methods
Today, galvanizing of metal is carried out using the following methods:
- hot;
- cold;
- galvanic;
- gas-thermal;
- thermal diffusion.
The choice of method that will be used for galvanizing parts and structures made of steel depends on their operating conditions, as well as on the characteristics that the protective layer must meet. Regardless of the galvanizing technology used, it is necessary to determine in advance the thickness of the protective layer being formed, which depends on such technological process parameters as the time of exposure of the metal to the working environment, as well as on the processing temperature. When using steel parts and structures on the surface of which a layer of zinc coating is applied, it should be borne in mind that they should not be subjected to significant mechanical stress, since the protective coating of this metal is highly brittle and can easily be destroyed.
To understand which types of galvanizing should be used in a given situation, you need to study each of them well.
Hot galvanizing
Hot galvanizing of metal structures, although it makes it possible to achieve the best quality and durability of processed products, ranks only second among similar technologies in terms of prevalence. When using this method, the problem of environmental safety arises, since its implementation requires the use of strong chemical reagents to prepare the surface to be treated, and the procedure itself is performed in molten zinc.
Industrial hot dip galvanizing line
In the process of galvanizing steel using the hot method, there are two stages:
- preparing the surface of the product for processing;
- the procedure itself for coating metal with zinc.
In turn, the preparation of the treated surface is also carried out in several stages:
- cleaning and degreasing;
- etching using acid solutions;
- washing after pickling and fluxing;
- thorough drying.
Immersion of supports in a hot zinc bath
After the product has gone through all the stages of preliminary preparation and has completely dried, it is placed in a special bath filled with molten zinc. As a result, a thin layer consisting of iron and zinc (Fe-Zn) is formed on the surface of the steel product, which provides reliable protection against corrosion. After removal from the bath, the product is blown with compressed air, which ensures not only its drying, but also the removal of excess zinc from the treated surface. The big disadvantage of this method of galvanizing metal is that the dimensions of the products that can be subjected to it are limited by the dimensions of the bath with molten zinc. Meanwhile, at large manufacturing enterprises, the process of galvanizing steel - scaffolding, lighting masts, power transmission line supports - is carried out in exactly this way.
Since this method is associated with high labor costs and the need to use complex technological equipment, it is not used for galvanizing metal at home.
Cold galvanizing
The wide popularity that the cold galvanizing method of steel has gained in recent years is due to a number of reasons. The most important of them is that, despite its high manufacturability and ease of implementation, this galvanizing method allows you to create a layer on the metal surface that has high protective properties. It is also important that this does not require equipment for galvanizing metal, so this galvanizing can be done with your own hands, even at home.
The essence of cold galvanizing technology is that a special zinc-containing mixture is applied to the surface of the product being processed, which can be zinconol or any other composition. You can apply zinconol or another mixture using a regular brush or roller. In cases where it is necessary to coat products with complex configurations or hard-to-reach places with such a mixture, you can use a spray gun to apply it. Zinconol and other compounds used for cold galvanizing make it possible to obtain a protective layer on the metal surface containing 89–93% zinc.
Cold galvanizing scheme
Galvanizing metal using the cold method has no alternative in cases where it is necessary to provide corrosion protection to structures that cannot be coated with a layer of zinc using other technologies. Such structures, in particular, include already installed pipes, power line supports, elements of railway tracks, as well as other metal elements that are in an installed (stationary) state.
Zinconol and other compounds for cold galvanizing are widely used in repair work when it is necessary to restore the damaged zinc layer on a metal product or structure. In particular, using this method, restorative galvanization of a car body can be performed (moreover, you can use zinconol and other mixtures for the initial, complete galvanizing of the body yourself).
Compositions for cold galvanizing are polymer solutions with the addition of highly dispersed zinc powder
Cold galvanizing of steel products can be carried out in a fairly wide temperature range, and the resulting coating is distinguished not only by high protective properties, but also by good elasticity, resistance to mechanical damage and thermal expansion.
If we talk about the disadvantages of the cold galvanizing method, they include the insufficiently high resistance of the formed coating to mechanical stress, as well as the need for strict adherence to safety precautions when carrying out such a procedure, which requires the use of organic solvents.
Galvanic method
Galvanic galvanizing, during which an electrochemical effect is applied to the surface of the processed product, makes it possible to obtain coatings that are not only highly accurate in thickness, but also have exceptional smoothness. This electrochemical galvanizing ensures the formation of a protective layer on the metal surface, the thickness of which is in the range of 20–30 microns.
Galvanic galvanizing allows you to adjust the thickness of the formed protective layer, while it is uniform and highly decorative. Due to the fact that when performing galvanic galvanization, the metal and the zinc that is applied to its surface are combined at the molecular level, the finished coating is characterized by exceptionally high adhesion to the base metal. Meanwhile, the degree of adhesion is influenced by the presence of fatty and oxide films on the surface of the processed product, which are almost impossible to completely remove (especially in mass production conditions).
The galvanizing line includes a full range of processing (from preparation to fixing)
Galvanic galvanizing is performed as follows. The structure to be processed and the zinc plates are placed in an electrolytic solution and then connected to the positive and negative contacts of an electrical current source. Due to the difference in electrical potential formed in this way, the plates begin to dissolve in the electrolyte, and zinc molecules rush to the surface of the workpiece, settling on it and forming a uniform protective layer.
The great advantage that galvanic galvanizing differs from other technologies is that it allows the formation of a protective layer on the surface of the product, characterized by exceptional decorative characteristics. The galvanizer can regulate the thickness of such a layer.
The most significant disadvantage of this method is its rather high cost, which consists not only of the cost of zinc plates and electrolyte. For example, used electrolyte, which contains a fairly large amount of hazardous waste, must be thoroughly cleaned before being sent to the sewer, which also seriously affects the cost of the method.
Thermal diffusion galvanizing
Thermal diffusion technology of galvanizing metal (TDZ), which is often called sherardization, was developed back in the 20s of the last century, but for a long time it was not used actively enough. Since the end of the last century, galvanizing metal using this technology has regained popularity.
The essence of this method of coating a metal product with zinc is that the workpiece, together with a zinc-containing dry mixture, is placed in a sealed container in which a high temperature is created - about 2600°. Under the influence of such a high temperature, zinc atoms transform into a gaseous state, which greatly facilitates their diffusion penetration into the surface layer of the workpiece. This galvanizing technology is used mainly in cases where it is necessary to form a protective layer on the surface of the metal being processed, the thickness of which exceeds 15 microns.
Installation for thermal diffusion galvanizing
Thermal diffusion coating of metal products with zinc, preparation for which is carried out in the same way as with hot-dip galvanizing, has a number of advantages, which include:
- complete environmental safety of the process, since it is carried out in a sealed container;
- almost complete absence of pores on the finished protective coating, characterized by high adhesion to the surface being treated;
- high protective ability of the coating obtained using this technology (5 times higher than that of a zinc layer formed by galvanic method);
- the ability to adjust the thickness of the zinc layer in a fairly wide range;
- preservation of even the complex shape and geometric parameters of a zinc-coated product;
- no need for special disposal of generated waste.
This is what products coated with zinc using the TDC method look like
This method of coating metal products with zinc also has its disadvantages, which include:
- not very attractive dirty gray color of the finished coating and lack of metallic shine;
- low productivity;
- the presence of zinc dust inclusions in the ambient air during such a process, which is harmful to human health;
- heterogeneity of zinc coating thickness.
Gas thermal spraying of zinc
To coat a metal sheet or three-dimensional part with a layer of zinc, you can use the gas-thermal galvanizing method. The essence of this method is that zinc, initially present in the dry mixture or wire, is sprayed onto the surface of the workpiece in a gaseous environment. It is optimal to use this technology in situations where a zinc layer must be applied to large-sized products that cannot be processed by other methods.
The principle of gas-thermal galvanizing
When using this method, coating a metal product with zinc occurs as follows. Particles of molten metal, hitting the surface to be treated, form a thin layer, reminiscent of scales in its structure. This coating, which is characterized by the presence of a large number of pores, is complemented by the application of paints and varnishes. The layer created as a result of this combination has protective properties that allow the metal product on which it is applied to be successfully used for a long time in quite aggressive environments (high humidity, constant exposure to sea and fresh water, etc.).
The parameters of zinc coatings applied by all of the above methods are regulated by the corresponding GOST, which specialists should be guided by.
Advantages and disadvantages of galvanizing body elements
Galvanization can hardly be called a panacea, but the advantages of this method of anti-corrosion treatment are obvious:
- a thin layer of zinc well protects ferrous metal from exposure to oxygen, moisture, and salts, preventing the spread of corrosion areas;
- galvanization, depending on the application method, can last on body parts for 5-25 years;
- Coating iron parts with zinc is more reliable and much cheaper than using tin.
Unfortunately, such protection is not eternal - on average, the zinc layer thins by 1-5 microns every year. Disadvantages include higher cost compared to treatment with a special primer. But if you do it yourself, the procedure will not be so burdensome financially.
How to perform the procedure at home
Galvanizing at home is carried out mainly by the electrochemical method or using cold galvanizing technology, which is explained by the simplicity of these methods. To perform galvanizing yourself using the electrochemical method, you must carefully prepare the surface of the workpiece. Such preparation consists of cleaning and degreasing, as well as acid etching and subsequent rinsing with water.
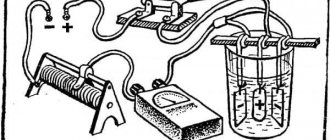
Diagram of a galvanic installation for self-galvanizing
Your own device for performing galvanic galvanizing can be made from a direct current source that produces a voltage of about 6–12 V with a current strength of 2–6 A, a container made of dielectric material and a device with which the electrode and the workpiece will be fixed. The electrolyte in this case can be a solution of any salt containing zinc. You can prepare such a solution from battery electrolyte by placing zinc in it for a while and waiting for the dissolution reaction to complete. The resulting composition should be filtered before use for galvanizing.
When performing galvanizing with your own hands, you should keep in mind that the following factors influence the thickness and quality of the coating formed:
- current density per unit area of the workpiece;
- temperature of the electrolytic solution used;
- density of the electrolyte used;
- geometric parameters and complexity of the shape of the processed product.
Painting a galvanized car body
How to paint a galvanized car body? This is a question many car owners ask. After applying the last layer of zinc, you must wait 24 hours for the solution to harden and the binder to evaporate, but be careful. It is recommended to wash the part with a solution of baking soda; in extreme cases, you can also use water to completely remove the acid from the element. Carry out the painting procedure in the usual way, and for this you need to apply a layer of primer, perform its finishing or fine polishing and evenly apply a layer of paintwork.
Is it difficult to check this yourself?
It is impossible to visually determine whether a machine or part of it is galvanized. But there are a few points that will help you figure this out:
- When purchasing a car, carefully inspect the body. 99% of the time you will find a couple of scratches. If the place where the paintwork was chipped simply darkened and there was no rust, the car or part of it was galvanized.
- Use the vehicle's VIN code, which contains technical characteristics, availability of protection and application method.
- Warranty card information. It says here about guarantees of anti-corrosion protection of the body. If a period of 5 to 10 years is indicated, this means cold galvanizing, 10-20 years - the galvanic method, more than 20 - the hot method.
- If the documents indicate that the car is galvanized, but the cost of the car is low, perhaps we are talking about either partial processing or the cold method.
If the machine has full anti-corrosion protection, the technical documentation indicates “full galvanization”.
Source