Metal anodization is an electrochemical process of creating a protective oxide film that protects the metal surface from environmental influences. Hence another name that best reflects the essence - anodic oxidation. Coating technology is used to process not only steel, but also most non-ferrous metals. The exceptions are iron and copper. These elements are characterized by the formation of two oxide compounds at once - this negatively affects the integrity of the film and its adhesion to the base surface.
During the development of anodizing, several methods of carrying out the work were developed. All of them will be discussed in detail in this article.
What is anodized metal surface
The name anodization is a process that occurs when using an electrolyte and electric current of various sizes and allows you to obtain a durable oxide foam on the product, which increases the strength of steel and provides protection against corrosion. Strength and mechanical characteristics vary depending on the composition of the metal, the density and type of electrolyte, the magnitude of the anodic and cathodic effects, calculated using special equations.
The protective coating itself is not applied, but is formed from the iron itself during an electrochemical reaction. The technology used at home looks schematically like this:
Scheme of the anodizing process at home
- Electrolyte is poured into a dielectric (non-conducting) container.
- A power supply is taken that can provide the required DC voltage at the output (this can be a battery or several batteries connected in electronic circuits).
- The “+” clamp is connected to the object being processed, and the object is immersed in a container with the solution.
- The “–” clamp is attached to a lead or stainless steel plate and is also lowered into the liquid.
- An electric current of the required magnitude is connected, according to the electrochemical equation. Thanks to it, oxygen begins to be released on the surface of the product, promoting the formation of a durable protective film.
Applicable devices and equipment
On an industrial scale, a sulfuric acid solution is used for anodizing steel, which ensures a high process speed and the greatest penetration depth.
Modern installations are fully automatic lines with a minimum number of personnel, whose role is reduced to monitoring the work process. All equipment can be divided into three types:
- Basics. It includes a bath and a cathode. The container must be made of an inert material with high thermal insulation properties - in this case, the electrolyte will not heat up too quickly and will last much longer. The cathode material depends on the type of metal being processed. For example, to anodize aluminum, a lead sheet is used, the size of which should be twice the dimensions of the workpiece.
- Serving. This includes units that are responsible for ensuring the operation of the installation: drive mechanisms and devices for transmitting current.
- Auxiliary. We are talking about equipment on which work is carried out to prepare workpieces for anodizing. This also includes mechanisms for moving parts and storing them.
When selecting a suitable installation, the following features must be taken into account:
- The most labor-intensive operations are loading and unloading the workpiece. Pay attention to the reliability and power consumption of these nodes.
- Productivity depends on the power of the power plant. As practice shows, the optimal rectifier power is 2.5 kW. The presence of stepless voltage level adjustment will be an additional advantage, facilitating the process of anodizing steel.
Stepless regulation will occur after the formation of a protective layer of medium thickness, when in order to maintain the current level it will be necessary to gradually increase the voltage.
- Contact pads made of flexible material should be installed along the rings of the container. Copper elements will cope best with this task.
Advantages of anodized metal
Anodic oxidation (anodizing) of various metals, carried out at home, is, of course, much inferior to that carried out using industrial equipment. But, nevertheless, it can provide the product with a number of advantages:
- Increase resistance to corrosion - due to the fact that the oxide film prevents moisture from penetrating the metal base, providing reliable protection. The use of this process on quickly rusting household items or disks and parts of household appliances can significantly extend their service life.
- Increase the strength of metal and steel: the oxidized coating is much more resistant to mechanical and chemical damage.
- Dishes treated in this way are non-toxic, resistant to prolonged heating, and food does not burn on them.
- After anodizing treatment, metal products acquire dielectric properties (they do not conduct current at all or almost not).
- Possibility of electroplating of another metal (chrome, titanium). Made with your own hands, it can significantly increase the mechanical strength characteristics or improve the decorative qualities (gold plating).
In addition, the process allows for decoration. You can do color anodic oxidation. This result can be obtained by changing the equations of the applied current and the density of the electrolyte (this is possible when anodizing titanium and other hard materials) or by using paint (more often for aluminum and other soft metals, but this process is also used on hard substrates). Objects painted in this way have a more even and deeper color.
The industrial method provides higher coating strength, the ability to carry out deep anodizing with the simultaneous application of cathodic electrochemical foam, which provides additional protection against corrosion. But even anodic-cathode treatment carried out at home will help make disks or other parts of moving mechanisms more durable and wear-resistant.
Anodizing stainless steel
One of the important tasks in preserving metal structures is combating the harmful effects of the environment. High humidity and the presence of chemically active elements in the air that can destroy the integrity of metal, especially steel, lead to a deterioration in such indicators as reliability and strength.
To solve this problem, finished products are coated with various types of protective coatings.
Steel oxidation
There are various methods to improve surface stability and corrosion resistance.
One of these methods is to create a protective film on the surface of steel using special processing methods.
Understanding the essence of the purpose of this process requires an answer to the question - what is oxidation?
The essence is to use the properties of the oxidation-reduction reaction, as a result of which a protective film is formed on the surface of the steel. Steel oxidation is also carried out.
This process allows you to solve the following problems:
- Protect steel structures from corrosion (this is especially important in modern construction where metal structures are used).
- Limit exposure to aggressive components of the external environment (solutions of acids, alkalis, chemical elements that destroy the integrity of steel).
- Create a surface layer with good electrical insulating characteristics.
- Give details, individual elements, and the structure as a whole original decorative and aesthetic properties.
Metal oxidation is carried out using the following methods:
- Using chemical reactions (chemical oxidation of steel).
- Use of electrochemical processes (anodic oxidation).
- Carrying out heat treatment (thermal method).
- Creation of low-temperature plasma (plasma method).
- Laser (special laser installations are used).
Anodized steel
Let's look at each method in more detail.
Different ways
There are two ways to carry out the process of oxidizing steel at home. Each of them has its own disadvantages and advantages .
Warm method
The easiest process to do it yourself. It runs successfully at room temperature, when using organic paint, and allows you to create amazingly beautiful things. For this purpose, you can use both ready-made paints and pharmacy dyes (green paint, iodine, manganese).
Hard anodizing cannot be achieved using this technology; the oxide foam is weak, provides poor protection against corrosion, and is easily damaged. But, if you paint the surface after this technique, the adhesion (adhesion) of the coating to the base will be very high, nitro enamels or other paints will adhere firmly, will not peel off, and will provide a high degree of protection against corrosion.
Cold method
This technique, when carried out at home, requires careful control of temperature, allowing it to fluctuate from –10 to +10°C (the optimal temperature for carrying out an electrochemical reaction according to the equation is 0°C). It is at this temperature regime that anodic and cathodic surface treatment occurs most fully, slowly creating a durable protective oxide film. This allows the home craftsman to carry out hard anodizing with his own hands, providing the steel with maximum protection against corrosion.
Using this method, you can do electroplating by applying copper, chromium or gold to the product, calculating the current strength using special equations. After such treatment, it is very difficult to damage the steel part or discs. Corrosion protection is effective for many years, even in contact with sea water, and can be used to extend the life of diving equipment.
A small disadvantage is that the paint does not adhere to such a surface. To give color to the metal, the sputtering method (copper, gold) or electrochemical color change under the influence of electric current is used (the current strength and density of the electrolyte are calculated using a special equation).
Anodic oxidation technology
The whole process, carried out with your own hands, can be divided into stages:
- The surfaces of disks and other metal parts are well cleaned of dirt, washed, and polished.
- Degreasing is carried out with white spirit or acetone.
- The required time is maintained in the alkaline solution (it is calculated by the equation based on the structure of the material).
- After this, the disks or other metal products are immersed in an electrolyte, where the anodic and cathodic reaction of growing an oxide film is carried out.
- If the product was cold processed, then after removing it from the container it should be thoroughly rinsed from acid and dried. After completion of this process, it is provided with long-term reliable protection against corrosion.
- During the thermal process, the film will be porous, soft, requiring additional strengthening, carried out by dipping in clean boiling water or by exposure to hot steam. Then you need to rinse it well.
How to consolidate the result
The quality of anodizing a metal such as aluminum will depend on the final stage - fixing the coating. To do this, after coating and washing, the element is placed in a manganese solution for ¼ hour. After removal, rinse the parts under hot and cold water to remove any remaining solution from the pores. Before dyeing begins, the microscopic pores on the film should be plugged, and for this, the product is boiled in distilled water for about 30-40 minutes.
Source
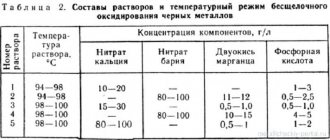
Types of electrolytes
At home, not only industrial chemical acid solutions are used, but also simple products that can be found in any kitchen:
- When anodizing titanium, you can take sodium chloride, sulfuric or phosphoric acid.
- For aluminum, oxalic, chromic or sulfuric acids are used.
- Instead of acids, you can use table salt and baking soda for anodic and cathodic treatment of disks or other steel objects. You can make the necessary electrolyte by mixing 9 parts of a concentrated soda solution with one part of saline.
The exposure time of disks, plates, and other metal objects in an electrolyte container under current is calculated according to the equation, based on physical and chemical parameters.
Dangerous moments
When using acids as electrolytes, safety precautions must be strictly observed. Neglecting them can lead to accidents:
- In case of contact with skin, due to the fact that the drug is diluted, minor burns are possible. But such a concentration is dangerous for the eyes, so you should not neglect protective glasses and gloves.
- Under the influence of current, oxygen and hydrogen vapors are released, which, when mixed, form an explosive gas. When working in a poorly ventilated area, you can get an explosion from any spark, which can be fatal.
By following safety precautions and the stages of technological processing, you can get durable, beautiful things: chrome plating car wheels, creating gold-look jewelry, adding strength to parts of household mechanisms, depending on the technologies used.