When laying an insulated pipeline in a trench and then backfilling it, the insulating coating may be damaged, and during the operation of the pipeline it gradually ages (loses its dielectric properties, water resistance, adhesion). Therefore, for all installation methods, except above-ground, pipelines are subject to comprehensive protection against corrosion with protective coatings and electrochemical protection (ECP) means, regardless of the corrosive activity of the soil.
ECP means include cathodic, sacrificial and electrical drainage protection.
Protection against soil corrosion is carried out by cathodic polarization of pipelines. If cathodic polarization is carried out using an external direct current source, then such protection is called cathodic, but if polarization is carried out by connecting the protected pipeline to a metal that has a more negative potential, then such protection is called sacrificial.
Cathodic protection
The schematic diagram of cathodic protection is shown in the figure.
The source of direct current is the cathodic protection station 3, where, with the help of rectifiers, the alternating current from the along-route power line 1, entering through the transformer point 2, is converted into direct current.
The negative pole of the source is connected to the protected pipeline 6 using connecting wire 4, and the positive pole is connected to the anode grounding 5. When the current source is turned on, the electrical circuit is closed through the soil electrolyte.
What are corrosion inhibitors
Corrosion inhibitors are substances that, when present in a corrosive environment in sufficient concentration, greatly slow down or even stop the corrosion destruction of metal. A corrosion inhibitor can be either a single compound or a mixture of several.
The effectiveness of corrosion inhibitors can be assessed by two indicators: the degree of protection (Z, %) and the corrosion inhibition coefficient γ (the protective effect of the inhibitor).
Formula for determining the degree of protection Z:
Z = [(K1 – K2)/K1]•100 = [(i1 – i2)/i1]•100,
where K1, K2 – rate of corrosion (dissolution) of the metal in a medium without and with an inhibitor [g/(m2•h)];
i1, i2 – corrosion current density in a non-inhibited and inhibited environment, respectively [A/cm2].
The Z value is 100% when the metal is completely protected, the corrosion rate is reduced to 0.
The protective effect of the inhibitor is calculated using the formula:
γ = K1/K2 = i1/i2.
The inhibition coefficient shows how many times the corrosion rate decreases under the influence of the inhibitor.
There is a relationship between the braking coefficient and the degree of protection, which is determined by the formula:
Z = (1 – 1/ γ)•100.
Schematic diagram of cathodic protection
1 - power lines; 2 - transformer point; 3 — cathodic protection station; 4 - connecting wire; 5 - anodic grounding; 6 - pipeline
The operating principle of cathodic protection is as follows. Under the influence of the applied electric field of the source, the movement of half-free valence electrons begins in the direction “anode grounding - current source - protected structure”. Losing electrons, the anodic grounding metal atoms pass in the form of ion atoms into the electrolyte solution, i.e. the anodic grounding is destroyed. Ion atoms undergo hydration and are removed into the depth of the solution. At the protected structure, due to the operation of the direct current source, an excess of free electrons is observed, i.e. conditions are created for the occurrence of oxygen and hydrogen depolarization reactions characteristic of the cathode.
Underground communications of oil depots are protected by cathode installations with various types of anodic grounding. The required protective current strength of the cathode installation is determined by the formula
Jdr=j3·F3·K0
where j3 is the required value of the protective current density; F3 is the total contact surface of underground structures with the ground; K0 is the coefficient of exposure of communications, the value of which is determined depending on the transition resistance of the insulating coating Rnep and the electrical resistivity of the soil rg according to the graph shown in the figure below.
The required value of the protective current density is selected depending on the characteristics of the soil at the oil depot site in accordance with the table below.
Corrosion protection
Protection against corrosion Methods of protection against corrosion can be combined into the following groups: 1) application of protective coatings and films; 2) change in the electrochemical potential of the protected material in relation to the medium at the phase boundary; 3) modification of the corrosive environment. Fighting corrosion using protective coatings is the most common method. Metal and non-metallic coatings are used as protective coatings. Metal coatings can be made of a metal more or less noble than the substrate. In this regard, they are divided into two groups: cathode and anodic coatings. Cathodic coatings include those coatings whose electrochemical potential under given conditions is greater than that of the metal being protected. Aluminum is almost always coated with cathodic coatings. Precious metal coatings on steel have the same character. Cathodic coatings protect the metal only by isolating it from the attacking environment. Therefore, they fulfill their role only if there is complete continuity. If a gap forms in the cathode coating, then under corrosion conditions it becomes a cathode, and the open part of the protected metal becomes an anode element. The anodic surface is significantly smaller than the cathodic surface. Electrochemical destruction of metal is concentrated on a small surface. Taking into account the dangers hidden in possible discontinuities in cathode coatings, they are made of relatively large thickness. Anodic coatings are coatings made of metal whose electrode potential is lower than that of the metal being protected. For iron operating in slightly acidic or neutral solutions, the anodic coatings are zinc and aluminum. The protective properties of anodic coatings consist not only of mechanical isolation of the metal from a corrosive environment. They also consist of electrochemical effects. If the coating is damaged and a corrosive element is formed, the protected metal, which is the cathode, is not destroyed. Small discontinuities in anodic coatings are not dangerous. Metal coatings are applied by electrodeposition, immersion in molten metals, sputtering metallization, chemical salt deposition, diffusion, etc. Recently, vacuum coating has become increasingly common. Non-metallic coatings are used in the event of a chemical reaction of metal in appropriate environments. These include, in particular, aluminum oxide coatings obtained through a special electrolytic process. Phosphate coatings are used in most cases with additional protective media, such as paints, varnishes, etc. Phosphating steel consists of immersing the product in a dilute solution of phosphoric acid and acid phosphates of zinc or magnesium. As a result of the reaction, insoluble iron phosphate is formed, which during the process densely coats the surface of the metal. This group also includes ceramic coatings and vitreous enamels. These coatings are quite resistant to mineral and organic acids. Their disadvantage is increased fragility and low resistance under conditions of sudden temperature changes. Organic coatings include a variety of paints and varnishes. Knowledge of the corrosion mechanism has made it possible to create methods of corrosion protection by applying a potential to the metal at which it becomes thermodynamically stable. Such methods include cathodic protection and reducing the aggressiveness of the environment surrounding the metal structure. Cathodic protection consists of attaching an anode-protector with a more negative electrochemical potential to the protected structure. The protector (Latin protector - patron, protector) serves as such an anode, preventing the destruction of the protected alloy; The protector itself gradually deteriorates due to corrosion. The protector can be any metal that has a more negative potential in relation to a given alloy. However, the difference in potentials should not be too large so that the electrochemical process does not cause rapid destruction of the tread. Protectors are usually small plates attached to the protected part with rivets or bolts. Cathodic or sacrificial protection is widely used in the protection against sea and underground corrosion of metal structures, communications, pipelines, vessels, etc. Magnesium or zinc alloys are usually used as protector anodes to protect steel products. Protection can also be achieved by connecting the protected metal to the negative pole of the DC current. To reduce the aggressiveness of the environment, additives are introduced into it, called corrosion inhibitors , which either contribute to the passivation of the metal or significantly reduce the rate of its corrosion. The condition for the use of inhibitors is the operation of the product in a closed environment of constant composition. There are anodic and cathodic inhibitors. Various substances that form insoluble compounds in the anodic areas are used as anodic corrosion inhibitors. One of these passivators is chromium K2Cr207, introduced in an amount of 2 - 3 g/l into the coolant solution. Cathode inhibitors inhibit the cathodic process. These include various etching additives added in an amount of 1 - 2% to acids to remove scale without destroying the base metal. Volatile inhibitors, such as sodium nitrate NaNO2, are used to impregnate paper in which parts to be stored or transported are wrapped. As they evaporate, they saturate the space surrounding the parts, creating a protective gas environment. Volatile inhibitors are highly effective. Steel products wrapped in NaNO2 treated paper in 85% relative humidity conditions do not rust for 5 years. The advantage of volatile inhibitors is the elimination of the use of protective coatings, ease of re-preservation and constant readiness of parts for immediate use without additional processing. Anti-corrosion protection of fasteners
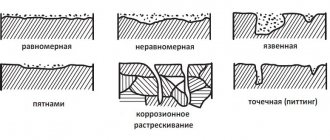
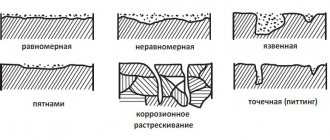
Corrosion is the process of destruction of metals during their chemical, electrochemical or biochemical interaction with the environment. The corrosion process is accompanied by the oxidation of the metal and its transformation into various chemical compounds (oxides, hydroxides, carbonates, etc.). Ferrous metals - carbon steel, cast iron - are most susceptible to corrosion, while many non-ferrous metals, alloy and stainless steels are very resistant to atmospheric conditions and aggressive environments. Increasing demands on the level of durability and corrosion resistance of machines and building structures lead to the emergence of more advanced methods and technologies for protecting surfaces from the destructive effects of the environment and aggressive operating conditions. Almost all machines, mechanisms, and building structures contain fasteners, which are subject to the same requirements for corrosion resistance and durability. The use of fasteners made of non-ferrous metals, stainless and alloy steels and alloys fully satisfy these requirements. However, the use of these materials is not always economically profitable and technically possible. In this regard, protective metal and non-metal coatings have become of great importance in the production of products and equipment. The use of such coatings, which protect carbon steel products from corrosion, reduces the consumption of expensive metals and alloys and reduces the cost of the final product. Types of anti-corrosion protective coatings. Based on the type of connection between the protective layer and the base, adhesive and diffusion coatings are distinguished. The formation of an adhesive coating is carried out due to the mechanical adhesion of particles of the coated layer to the rough surface of the base. The corrosion resistance of these coatings is largely determined by the strength of adhesion (adhesion) of the protective layer to the base. During the formation of a diffusion coating at the level of sections of the base material and coating material, processes of diffusion and chemical interaction occur. As a result of this process, a coating of complex composition is formed on the surface of the product, connected to the base by a diffusion zone, consisting of atoms of the coating material and the base material. Based on the type of anti-corrosion protection, a distinction is made between coatings that implement barrier protection and coatings that implement electrochemical protection. With barrier protection, the coating creates a barrier to the environment's access to the base material. Very sensitive to mechanical damage. Electrochemical protection creates interfaces between dissimilar metals with different electrode potentials in a given operating environment. To ensure reliable protection against corrosion of parts, a coating is used that, together with the metal of the product, will serve as an anode. Thus, for steel products, anodic coatings are zinc, cadmium, and aluminum. If moisture penetrates into the pores of the coating or into places where it is damaged and a corrosion process begins, it is not the metal of the part that will be dissolved, but the coating. Anti-corrosion protection of fasteners has its own nuances and features, which we will consider below. Historically, in world practice, it has developed that zinc-based coatings have become most widespread due to the optimal cost/protective properties ratio. Zinc has a more negative (0.2-0.3 mV) stationary electrode potential than iron (steel) and when exposed to aggressive environments (in the form of electrolytes) it slowly dissolves due to electrochemical reactions with the constant renewal of protective passive films, thereby protecting most, the main material of the fastener. Thus, the zinc coating acts not only as a barrier, but also as an electrochemical protection. Today in the world there are several technologies for the production and application of zinc coatings. Electroplating
Today, the most common technology for applying zinc coating to fasteners is galvanic galvanizing. Galvanic coatings are applied to the surface of a steel product by deposition of metals during electrolysis of aqueous solutions of the corresponding salts (electrolytes). The electroplating process is quite simple. It consists in the fact that the protected products with a prepared surface and a metal plate that needs to be applied as a protective coating are immersed in a solution of salts of this metal. The product to be coated serves as one electrode, and the plate of the deposited metal serves as another, and a direct current is passed between them. In this case, the coated product is the cathode, and the plate of the deposited metal is the anode. During the coating process, the anode dissolves, and from the solution the metal is deposited on the cathode (protected product), forming a galvanic coating with a thickness of 5 to 25 microns. A soluble anode is sometimes replaced with an insoluble one (for example, lead), in which case it is necessary to maintain a given electrolyte concentration. The resulting coating is adhesive. The adhesion of galvanic coatings is ensured by molecular forces acting between the molecules of zinc and the base metal. Galvanized products are brightened and passivated in special solutions to increase their corrosion resistance and improve their appearance. Galvanized springs and parts that are subject to mechanical deformation after coating (bending, flaring, stretching, etc.) must be dehydrated in air at a temperature of 180-200 ° C for 2 hours. After dehydration, the color of the passive film changes. It is allowed to carry out dehydration before clarification and passivation. In this case, after dehydration, the parts are treated in sulfuric acid (5-15 g/l) at a temperature of 15-30 °C for 3-5 minutes. Coatings of steel products with zinc, cadmium, tin, chromium, nickel and lead are most often used in industry and construction. The figure shows the microstructure of the galvanized zinc coating on a screw with a diameter of 4 mm. Here you can see the adhesion area (dark stripe) between the base and the electroplated coating and porosity. After applying the coating, for greater stability and durability, it is subjected to clarification - activation of the coating surface with nitric acid and passivation (chromatization) - the creation of an additional passive protective layer (also called a conversion coating) on the surface of the protective coating itself.
Passivation can be rainbow (yellow), colorless (white or blue), olive and black. During passivation, most enterprises use hexavalent chromium, a carcinogen and poison. The main disadvantages of such coatings are the loss of corrosion resistance when heated above 100°C and the environmental unacceptability of the technology. Since the beginning of 2007, there has been a ban on the use of hexavalent chromium in passivation films in the automotive industry.
Replacing chromate films with chromite films that do not contain hexavalent chromium solves the identified problems. Passivation with trivalent chromium is already used by some manufacturers of automotive fasteners; in this type of coating, they satisfy current standards for corrosion resistance, but are assessed by experts as unpromising. The use of passivating solutions and electrolytes containing acids, cyanides and other chemically active compounds forces the organization of methods for neutralization and deep purification of environmentally hazardous waste in electroplating industries, and the construction of expensive treatment facilities, which ultimately neutralizes the positive qualities of high-performance electroplating processes. Therefore, such coatings, along with phosphate, oxide and some others, are called conversion coatings. Due to their barrier and, in some cases, electrochemical protective properties, they significantly increase the corrosion resistance of galvanic coatings. There is a wide variety of conversion films on zinc coatings: colorless, rainbow, olive, black, which differ not only in appearance, but also in corrosion resistance, composition of working solutions, and processing mode (Fig. 2). Advantages and disadvantages Electroplating has a number of advantages and disadvantages. Advantages: • coating of high chemical purity; • electrochemical or barrier protection; • good decorative properties; • the ability to adjust the thickness of the coating with acceptable accuracy; • do not require special preparation of threaded parts of products. Disadvantages: • porosity; • low wear resistance; • low durability; • deterioration of the mechanical properties of fasteners made of alloy steels due to hydrogenation of the base material; • lengthy coating process; - high toxicity and complex waste disposal process. Hot-dip coating ( hot-dip galvanizing )
The method of applying a coating by immersion in a melt is based on the diffusion interaction of the base metal with the molten coating metal at the interface between two phases. The formation of the coating is carried out through contact interaction of the liquid phase - coating metal with the solid phase - base metal. In this case, at the interface between the two phases, the processes of wetting, mutual diffusion and, after removing the coated product from the melt, crystallization occur. The resulting coatings have a complex structure. The layer adjacent to the steel consists of intermetallic compounds of the coating metal and the base, and the surface layer, which determines the durability of the coating, is made of hardened coating metal.
Hot-dip galvanizing technology includes degreasing steel products in alkaline solutions, chemical etching in acid solutions and fluxing, most often in solutions of zinc and ammonium chlorides, followed by drying, after which the products are placed in a drum and dipped into a bath (usually ceramic) with molten zinc . Rotation of the drum ensures the flow of zinc relative to the products to fill all pores and microcracks. The drum is then removed from the bath and spun to remove excess zinc by centrifugation. However, excess zinc still remains on the internal threads (on the nuts) (Fig. 4), so the internal threads are re-ground. The absence of a coating on the internal thread does not affect the corrosion resistance of the connection if the nut is used with a hot-dip galvanized counter part - a bolt or stud. Due to the high anodicity of zinc in relation to iron at temperatures up to 70°C, zinc itself covers damaged areas of the part at a rate of about 2 mm per year. In this case, zinc from the external thread of the bolt, due to the potential difference between zinc and iron in a natural humid and acidic environment, is transferred to the areas of the internal thread of the nut that remain when the thread is turned without coating. Advantages: • corrosion resistance is 5-7 times higher than the resistance of galvanic coating; • electrochemical and barrier protection; Disadvantages: • porosity; • poor decorative properties; • special preparation of threaded parts of products is required; • greater, in comparison with galvanic coating, coating thickness of 40-60 microns; • low wear resistance; • possible deterioration of the mechanical properties of fasteners made of alloy steels due to exposure to high temperatures; • lengthy coating process; • high toxicity and complex waste disposal process.
Protective anti-corrosion coating Delta-MKS® ( zinc lamellar ).
Zinc lamellar coatings are a type of non-electrolytic coating using finely dispersed zinc and an electrically conductive binder. The name of this coating comes from its filler - dispersed zinc, which is presented in the form of small flakes (lamellas) a few tenths of a micron thick, with a width (length) of 20÷30 microns. One of the most famous in the world and at the same time represented on the Ukrainian market is the German technology for applying zinc dispersed anti-corrosion systems Delta-MKS®. The protective system of zinc-lamella coatings Delta-MKS® consists of a base layer and an additional insulating layer, which, along with anti-corrosion protection of metal products, provides additional properties - frictional, elastic-plastic, thermal, chemically resistant, mechanical, decorative, etc. Base layer - This is an electrically conductive matrix of inorganic origin (varnish) with parallel zinc flakes located in it that have undergone passivation without the use of Cr(VI) chromium. The base layer, due to electrical conductivity and the presence of dispersed zinc, is an anode in relation to the protected surface of the steel part, since the electrode potential of zinc is more electronegative in value than the electrode potential of iron (steel), the process of cathodic protection is realized. Thus, in the event of external mechanical damage to the coating, zinc particles first undergo corrosion. And only after complete corrosion of the zinc does the steel part begin to corrode. The thickness of the base layer can be in the range of 5÷10 microns. An additional protective layer is an organic solvent-based varnish (high network polymer) applied to the base layer. In fact, this is additional cathodic-type anti-corrosion protection with electrical insulating properties. This additional layer protects the base layer from the occurrence of “white corrosion” - corrosion of zinc, isolating it from environmental influences. In addition, the additional layer allows the surfaces of parts to be painted in a wide range of colors and can serve as a carrier of an integrated lubricant that allows you to adjust the coefficient of friction in threaded connections. The thickness of the finishing layer can be in the range of 3÷5 microns.
Application technology
Legend
The Delta-MKS® coating technology includes the following main stages: • Preparatory - step-by-step washing in aqueous solutions of detergents followed by shot blasting to activate the surface layer of products. • Coating is the cyclic immersion of fasteners in a solution followed by centrifugation, thereby removing excess solution on the products. • Heat treatment – preheating to a temperature of 60–80° C. Conditioning of coated fasteners in a continuous furnace at a temperature of 180–250° C. • Cooling – forced cooling and unloading. The processes can be repeated several times until the required coating thickness is obtained.
Advantages and disadvantages
Advantages: • electrochemical or barrier protection; • extremely thin thickness, usually 4 – 12 microns; • temperature influence on the product material is excluded, maximum temperature during application is 250°C; • eliminates hydrogenation (saturation with hydrogen) of the surface layer of products made of high-strength steels;
• attractive appearance of products, it is possible to integrate color into the finishing layer; • friction coefficients for threaded parts are set in accordance with customer requirements, in addition they satisfy other properties of bolted connections • do not use heavy metals that are hazardous to health, such as chromium (VI); • have high resistance to chemicals and organic solvents; • withstands from 6 to 10 cycles of tightening a threaded connection at extreme torques; • maximum constant temperature of use up to 200 ˚C; • Suitable for flexible and elastic elements such as spring washers, Grover washers, coil and disc springs, etc. due to elasticity; • allows you to adjust the coefficient of friction in a threaded connection within µ=0.09-0.18 due to the solid lubricant integrated into the base or additional layers. This advantage allows, along with highly effective anti-corrosion protection, a stable torque coefficient of fasteners, which is very important for high-quality installation of critical metal structures and other connections. In our opinion, this coating has no disadvantages. Disadvantages: Low electrical conductivity of connections due to the use of organopolymer coatings. Limited temperature range of use maximum 260˚C. Coating of small parts. Due to technological features, it is impossible to guarantee the uniformity and stability of the coating on small parts, parts with fine threads, etc. By evaluating the corrosion resistance of Delta-MKS® zinc lamel coating using the neutral salt spray test in accordance with ASTM B117/DIN 59021 and analyzing the values according to DIN 50961, comparative data were obtained :
| № | Name of coating | Time before corrosion appears, hour | Approximate service life of coatings in urban conditions, year |
| 1. | Electroplated zinc + chromating, 5 microns | 40÷45 | 2÷3 |
| 2. | Electroplated zinc + chromating, 8 microns | 100÷120 | 3÷4 |
| 3. | Hot zinc, 40÷60 microns | 380÷400 | 10÷12 |
Tread protection
The principle of operation of the tread protection is similar to the operation of a galvanic cell.
Two electrodes: pipeline 1 and protector 2, made of a more electronegative metal than steel, are lowered into the soil electrolyte and connected by wire 3. Since the protector material is more electronegative, under the influence of a potential difference, a directed movement of electrons occurs from the protector to the pipeline along the conductor 3. At the same time, the ion atoms of the protector material go into solution, which leads to its destruction. The current strength is controlled using control and measuring column 4.
Methods for protecting pipelines
Corrosion of pipelines occurs during their operation. Rust formation occurs on pipes inside and outside. Deposits appear on the inside, and the reason for this is chemical reactions between the composition of the transported liquid and the metal. The condition of the surface is also influenced by high soil moisture.
If protection is not provided in a timely manner, a number of consequences may occur. What is important:
- It is recommended to carry out routine inspections at short intervals.
- Carry out repair work periodically, regardless of the presence of corrosion.
- suspension of the functioning of pipeline transport is inevitable, since it is necessary to carry out inspections and carry out scheduled preventative and other routine repairs.
Important! To ensure complete protection, it is necessary to take into account the installation method, contact with aggressive media, and the type of pipeline.
Dependence of protective current density on soil characteristics
| Soil type | rp ohmm | A, A/m2 |
| Wet clay soil: | ||
| — pH >8 | 15 | 0,033 |
| pH = 6-8 | 15 | 0,160 |
| - mixed with sand | 15 | 0,187 |
| Wet peat (pH <8) | 15 | 0,160 |
| Moistened sand | 50 | 0,170 |
| Dry clay soil | 100 | 0,008 |
Classification of corrosion inhibitors
Corrosion inhibitors are classified according to the type of medium:
— inhibitors of neutral corrosive environments;
— atmospheric corrosion;
— inhibitors of acidic environments;
— hydrogen sulfide corrosion;
— oil media inhibitors.
In different corrosive environments, the same inhibitor can behave completely differently.
Classification of corrosion inhibitors by mechanism of action:
— passivating inhibitors;
- adsorption inhibitors.
According to the nature of the protective action, inhibitors are distinguished into anodic, cathodic, and mixed.
According to their chemical nature, inhibitors are divided into: volatile, organic, inorganic.
Adsorption corrosion inhibitors are adsorbed on the surface of the protected product, forming a film, and inhibit electrochemical reactions. Sometimes the formation of a thin monomolecular film is sufficient. Adsorption inhibitors are most often surfactants (surfactants), as well as organic compounds. When exposed to the product, they further enhance the protective properties of the oxide film. Therefore, we can conclude that the presence of oxygen in a corrosive environment helps to increase the protective effect of adsorption corrosion inhibitors. If the oxide film is unstable, the adsorption of the inhibitor on the metal surface becomes difficult; oxygen has no additional effect.
Passivating corrosion inhibitors play an important role in the formation of a protective film on the metal surface, which passivates it. Inorganic compounds with oxidizing properties (nitrites, molybdates, chromates) most often act as passivators. When the surface is treated with these substances, the corrosion potential shifts to the positive side. Passivating compounds are considered more effective than most non-passivating compounds.
Inorganic corrosion inhibitors are the most commonly used. These include some passivators, cathodic, anodic, film-forming inhibitors, etc. The inhibitory effect of such compounds can be explained by their composition. Some anions (PO43-, NO2-, CrO42-, SiO32-, Cr2O72-), as well as cations (Ni2+, Ca2+, As3+, Sb3+, Zn2+, Bi3+) help reduce the rate of the corrosion process.
Inorganic corrosion inhibitors include phosphates, bichromates, chromates, nitrites, polyphosphates, silicates, etc.
Organic corrosion inhibitors are considered mixed action substances. They slow down the cathodic and anodic reactions. They are often used in acid etching. In this case, various contaminants, rust, and scale are removed from the surface, but the base metal does not dissolve. The protective effect of organic inhibitors depends on their concentration, temperature, and the nature of the compounds.
Most often, organic inhibitors include oxygen, nitrogen, and sulfur. They are adsorbed exclusively on the metal surface. Organic inhibitors include some volatiles, amines, organic acids and their salts, mercaptans (thiols), etc.
Schematic diagram of tread protection
1 - pipeline; 2 — protector; 3 - connecting wire; 4 - control and measuring column
Thus, metal destruction still occurs. But not the pipeline, but the protector.
Theoretically, to protect steel structures from corrosion, all metals located in the electrochemical voltage series to the left of iron can be used, since they are more electronegative. In practice, protectors are made only from materials that meet the following requirements:
- the potential difference between the tread material and iron (steel) should be as large as possible;
- the current obtained by electrochemical dissolution of a unit of mass of the protector (current output) must be maximum;
- the ratio of the tread mass used to create protective current to the total loss of tread mass (utilization factor) should be the greatest.
These requirements are best met by alloys based on magnesium, zinc and aluminum.
Tread protection is carried out with concentrated and extended protectors. In the first case, the electrical resistivity of the soil should be no more than 50 Ohm-m, in the second - no more than 500 Ohm-m.
Electrical drainage protection of pipelines
A method of protecting pipelines from destruction by stray currents, providing for their removal (drainage) from the protected structure to a structure that is a source of stray currents or special grounding, is called electrical drainage protection.
Direct, polarized and reinforced drainage are used.
Combination of protectors and paints
Often there is a need to protect a gas pipeline from corrosion not only with a protector, but with paint and varnish material. Paint is considered a passive method of protection against corrosion processes and is truly effective only when combined with the use of a protector.
This combination technique allows:
- Reduce the negative impact of potential defects in the coating of metal structures (peeling, swelling, cracking, heaving, etc.). Such defects occur not only as a result of manufacturing defects, but also due to natural factors.
- Reduce (sometimes by a very significant amount) the consumption of expensive protectors, while increasing their service life.
- Make the distribution of the protective layer over the metal more uniform.
It is also worth noting that paint and varnish compositions are often not easy to apply to certain surfaces of an already operating gas pipeline, tanker or some other metal structure. In such cases, you will have to make do with only a protective protector.
Cathode, anodic, mixed inhibitors
In most cases, inhibitors protect the product from corrosion through an electrochemical mechanism, i.e. influencing the rate of cathodic, anodic, or both corrosion processes. The essence of inhibition is to slow down this speed.
Anodic corrosion inhibitors
Anodic inhibitory additives affect the anodic reaction. These are compounds that have an oxidizing effect (nitrites, chromates). They contribute to the formation of a very thin passive film on the anodic part of the metal product, which significantly slows down the corrosion rate in this area. Anodic inhibitors are also called passivators. The mechanism of action of anodic inhibitors: due to the formation of a passive film, the anodic surface area decreases; inhibition of the anodic transition of the base metal into solution.
Most anodic corrosion inhibitors are considered dangerous because... with an overdose or a lack of them in the solution, the opposite effect to the protective effect (increased corrosion rate) can be observed. Anodic inhibitors include phosphates, silicates, alkali metal carbonates, hydrogen phosphates and many others. When there are insufficient concentrations of anodic corrosion inhibitors in a corrosive environment, localization of corrosion processes and an increase in the rate of metal dissolution in certain areas are observed.
Cathodic corrosion inhibitors
Cathode inhibitors slow down the cathodic reaction, the dissolution of the metal. The stationary potential of the system shifts in the negative direction, and the corrosion current decreases. An adsorption film forms on the surface. A chemical reaction takes place, as a result of which the depolarizer binds. Hardly soluble compounds are formed on the surface of the protected metal, which slow down corrosion by blocking the surface. Cathodic inhibitors are less effective than mixed or anodic inhibitors, so their use is limited. Cathode, like anodic, are not used in acidic environments, because they are ineffective. These include sodium sulfite and hydrazine.
Neutral media inhibitors
According to Rosenfeld, inhibitors of this type are classified as follows:
- with oxidizing properties (chromates, sodium nitrite, organic compounds that contain nitro and carboxyl groups);
- inhibitors that form sparingly soluble compounds, but do not have oxidizing properties (borates, silicates, phosphates, sodium carbonate, sodium hydrate);
- inhibitors with a weak oxidative effect with anions such as (MetO4)n- (vanadates, chromates, tungstates, molybdates).
Several groups of commonly used neutral corrosion inhibitors are discussed below.
Sodium nitrite
The most widely used inhibitor of neutral media is the anodic inhibitor sodium nitrite NaNO2. An affordable, simple inhibitor is very often used to protect steel in water. As the temperature increases, the effectiveness of sodium nitrite decreases, so its concentration must be increased.
Very often, sodium nitrite is used for inter-operational metal protection. To do this, its surface is treated with a 10% aqueous solution of the inhibitor. The concentration of sodium nitrite largely depends on the amount of chlorine ions in the water. The concentration of this substance should be 10 times greater than the concentration of chlorine ions.
Sodium nitrite is not used to protect copper and zinc at pH values greater than 5.
Phosphates
Widely used to inhibit cooling systems of power plants. Phosphates are fairly strong inhibitors and are also non-toxic. They must be handled carefully so as not to overdo the concentration. If you introduce too much, the corrosion rate will, on the contrary, increase. Phosphates with corrosion products form poorly soluble compounds on the surface of the steel, which become compacted over time, isolating the surface. Phosphates, like sodium nitrite, are a dangerous inhibitor because if you introduce it into the system in too small or large quantities, this will lead to increased corrosion destruction. But phosphates have their own advantages over sodium nitrite - their protective effect does not depend on the content of chlorides in water. 10 mg/l is a commonly used phosphate concentration to protect steel in water.
Chromates
Chromates are universal inhibitors because used to protect almost all metals. Very effective for inhibiting aqueous media. In practice, they are often used to protect coolants from corrosion. The protective effect is greatly influenced by chlorine ions, which reduce the effect of the inhibitor. The concentration of chromates must exceed the concentration of chlorine ions by at least 2–3 times.
With increasing temperature, the effectiveness of chromate immediately decreases significantly; its greater concentration is required. For example, at a temperature of a corrosive environment of 20 °C, 2–3 times less inhibitor is required than at a temperature of 80 °C. If at elevated temperatures the corrosive environment contains an insufficient amount of inhibitory additives, the corrosion is local in nature.
Organic-based chromates (methylamine, cyclohexylamine, isopropylamine, guanidine) are considered slightly more effective.
Chromates are used only to protect metal in circulating water.
Among the inhibitors of neutral media we can distinguish: HEDP, NTA, FBTK, EDTA, NTP. These complex inhibitors (complexons) protect the product well only in hard water, forming compounds with magnesium and calcium cations.
For soft waters, IFKHAN-31 and 34 inhibitors are more suitable, which perfectly protect systems consisting of various metals and alloys.
Corrosion inhibitors for the gas and oil industries
The presence of various impurities and dissolved hydrogen sulfide in oil makes it a very aggressive, corrosive environment. Inhibition is used at all stages of oil production, refining, and storage. In this case, the same substance is used. Most inhibitory additives form a film on the metal surface and hydrophobize it. Paraffin inhibitors, antifoaming agents, and anti-ignition agents are also introduced into the oil. To protect metal from hydrogen sulfide corrosion (gas and oil industries), amine-based inhibitor additives have proven themselves to be effective.
Common inhibitory additives in the gas and oil industries: such as IFKHANGaz, sulfanol P, AMDOR-IK, Olazol-T2P, Corexit-6350, ISA-148, Sepakor 5478, Dodigen 4482-1, etc.