Aluminum is a soft metal that is easy to process. In this sense, it is very good for making various products, which largely explains its popularity. In addition to the positive aspects, there is one significant drawback of the metal - it oxidizes very quickly. A thin film on its surface seriously interferes with the process of painting the product, and unpainted metal looks unattractive. The problem can be solved by using aluminum anodizing.
The whole problem with the naturally formed oxide film, which, in principle, protects the metal from further destruction, is that it is fragile and easy to clean off. Anodizing helps build up a strong oxide film and bond it to the aluminum. After this, the metal can be painted or varnished, and these coatings will firmly adhere to the surface of the parts.
Principles of the anodizing process
The process of electrochemical oxidation of aluminum and its alloys in solutions of sulfuric, chromic, oxalic acids and their mixtures is called aluminum anodization. Despite its apparent simplicity, the anodizing process has many options that directly affect the characteristics and quality of the oxide film. The appearance and structure of the coating is also affected by the composition of the aluminum alloy, and adjusting the electrolyte allows you to change the properties of the coating within a wide range. The quality and presence of impurities in the electrolyte composition can also be critical.
Anodizing differs significantly from the processes of electroplating metals (electrochemical deposition), in which a protective or decorative layer of metal is applied to the surface of a metal product, as it is a process of transforming the base metal, as a result of which the appearance and characteristics of the surface change.
Cold anodizing
Technologically, the process is similar to the previous option, the only difference is that such anodization occurs at a low temperature, in the range from -10 to +10 °C. The advantage of this method is that the protective film is thick and durable. The cold environment acts so that the layer grows faster on the inside than it dissolves on the outside.
The processed product is highly resistant to corrosion. The technique has a drawback - it is almost impossible to qualitatively paint anodized metal with organic compounds.
Painting improves the quality and aesthetics of the surface Source gidpokraske.ru
Application of Anodizing
The use of anodization is a topic for a separate article; in any industry where products made of aluminum or its alloys are used to one degree or another and any properties of the metal need to be changed, anodization is the optimal and often the only solution.
Here is a list of the main areas of application of anodizing:
- Thin oxide films are used as a basis for applying organic and inorganic coatings (paint or varnish).
- Color anodizing. The use of various coloring electrolytes makes it possible to obtain a wide range of shades and colors of the surface of an aluminum product. Nickel, cobalt or tin salts are used as additives. The resulting shades range from light bronze to black.
- Increased wear resistance. Oxide coatings on aluminum are much harder than the base metal. Hard anodizing is widely used for parts subject to abrasion under light loads, as well as to increase the corrosion resistance of products.
- Electrical insulation. Compared to organic insulating materials, oxide film not only has high insulating properties, but also has significantly greater heat resistance.
- Obtaining a compacted surface with high anti-friction properties. (lubricating coating).
Terms and concepts
First about terminology
For brevity, we will use “ anodic oxidation
" and "
anodic oxidation
" is a shorter, but with the same meaning, the term "
anodizing
", and instead of "GOST" "
anodic oxide coating
" - the simpler and more popular "
anodic coating
".
What is anodizing
Anodizing is a method of increasing the corrosion resistance of a metal product by forming a layer of oxide on its surface. The product being processed is the anode in this electrolytic process. Anodizing increases the product's surface resistance to corrosion and wear, and also provides greater adhesion to paints and adhesives than bare aluminum.
Anodic coatings can also be used as decorative coatings or as porous coatings that can absorb various dyes, or as transparent coatings that produce interference effects when light is reflected. Such interference coatings are used, for example, on bicycles or cyclists' clothing so that they can be clearly seen at night.
How does anodizing work?
The process of creating this protective oxide coating occurs electrolytically. The metal product to be coated with an anodic coating (usually aluminum) is immersed in a bath of electrolytic solution. Cathodes are installed in the same bath, usually along the sides of the bath. When an electric current passes through an acid solution, hydrogen is released at the cathode and oxygen at the anode. This leads to the fact that an oxide film begins to grow on the anode - the aluminum product.
Depending on the purpose of the anodic coating and the anodizing process used, it is possible to obtain an anodic coating with different characteristics. The anodic coating that can grow on an aluminum product can be 100 times thicker than the oxide coating that forms naturally on aluminum.
Since the metal piece is the "anode" in this electrolytic process, the entire process is called "anodizing."
Anodizing of metals
Although anodic coatings can also be formed on various metals, including titanium, hafnium, zinc and magnesium, anodizing generally refers to the anodizing of aluminum and its alloys.
Why anodize aluminum?
Aluminum's popularity is largely due to its good natural corrosion resistance. It is achieved due to the high chemical affinity of aluminum for oxygen, that is, their great mutual tendency to react with each other to form aluminum oxide. This very thin oxide film instantly coats any fresh aluminum surface as soon as it comes into contact with air. However, in some cases it is necessary to have a higher degree of protection (corrosion or chemical), modify the appearance of the surface (color, texture, etc.) or create specified physical properties of the surface (increased hardness, wear resistance or adhesion). In such cases, they resort to anodizing aluminum and aluminum alloys.
Figure 1 – Scheme of the anodizing process
Selecting anodizing electrolyte
As mentioned above, the properties of the oxide film obtained by anodization are influenced by many factors - the type of aluminum alloy, the method of pre-treatment of the surface of the part, the anodizing mode and the type of finishing operations. The composition of the electrolyte is also decisive. Acid electrolytes are mainly used (alkaline electrolytes can be used in some cases for special types of anodizing). The main acid is sulfuric; the vast majority of anodizing electrolytes are prepared on its basis. Other acids are used to obtain special types of coatings.
Types of anodizing
The QUALANOD organization divides aluminum anodizing into four main types with different requirements for their characteristics and properties:
- architectural (construction) anodizing
- decorative anodizing
- industrial anodizing
- hard anodizing.
Anodic coatings are divided into classes according to their thickness:
- minimum permissible average thickness and
- minimum permissible local thickness.
For example, class AA20 means that the average coating thickness must be at least 20 micrometers. The minimum local coating thickness should generally be at least 80% of the minimum average thickness. For class AA20 this is 16 microns.
Architectural anodizing
This is anodizing for the production of architectural finishing of products that are constantly exposed to outdoor conditions and in a stationary state. The most important characteristics of an anodized product are appearance and long service life.
For anodized aluminum, the degree of protection against pitting corrosion of aluminum increases with increasing thickness of the anodic coating. Consequently, the service life of architectural or building elements largely depends on the thickness of the anodic coating. However, obtaining a thicker anodic coating requires significantly more electrical energy. Therefore, so-called “re-anodizing” is not recommended.
Architectural anodizing has the following classes:
- AA10
- AA15
- AA20
- AA25
The choice of anodic coating thickness for external aluminum structures depends on the aggressiveness of the atmosphere and is usually specified in national regulations. In addition, the use of some colorants requires a thickness grade of 20 microns or higher. This is necessary to achieve good filling of the pores with the dye and increased resistance of the painted coating to sunlight.
Decorative
This type of aluminum anodizing is intended for the production of decorative finishing products. The main criterion for quality is a uniform or aesthetically pleasing appearance.
Decorative anodizing has the following standard thickness classes:
- AA03
- AA05
- AA10
- AA15
Industrial and solid
Industrial anodizing of aluminum is used to produce functional surface finishes for products when appearance is a secondary characteristic. The purpose of hard anodizing is to obtain a coating with high wear resistance or high microhardness.
Very often, for example in the automotive industry or medical equipment, the appearance of the product is not important, but the most important characteristic is resistance to wear and/or ability to be effectively cleaned and have high hygiene requirements. In such cases, it is these properties of anodized aluminum that are most important.
If the main property is high wear resistance, a special type of anodizing is used - hard anodizing. It is produced at low, often negative, electrolyte temperatures
Industrial and hard anodic coating thicknesses typically range from 15 to 150 microns. Threads and splines can be coated up to 25 microns. To obtain high electrical insulation, an anodic coating thickness of 15 to 80 µm is often required. Coatings with a thickness of 150 microns are used for repairing parts.
Anodizing in sulfuric acid electrolyte
Anodizing in sulfuric acid makes it possible to obtain translucent, colorless coatings with a thickness of about 35 microns. If the anodizing process is preceded by a process of glossing the surface of the parts, the coatings obtain high decorative qualities (shiny anodizing). In sulfuric acid, plastic anodic films are also obtained, which are not destroyed when molding products.
Sulfuric acid concentration and electrolyte temperature
The concentration of sulfuric acid for anodizing in industrial conditions is taken in the range of 8-35% (by weight). In a concentrated solution, the anodic film becomes soft and porous, and the elasticity of the film is high. The classic concentration is 15% (by weight). The temperature during the anodizing process is set in the range from 180C to 250C. In most cases, a temperature of 200C is accepted. Using sulfuric acid, solid anodic films are also obtained; in this case, the anodizing process is carried out at low temperatures (from -5 to +5 0C).
Temperature control during the anodizing process is mandatory; the current density and the rate of film dissolution depend on the temperature, which in turn has a direct impact on the quality and characteristics of the coating. In order to avoid local overheating of the electrolyte solution, special mixing devices are used.
Voltage and current density
When anodizing in sulfuric acid, a standard rectifier with an output voltage of up to 24 volts is used. In standard mode, the current is 16 volts with a current density of 1.5 A/dm2. To obtain corrosion-resistant films of large thickness, the voltage and current are raised to 18 volts, and when processing aluminum-silicon alloys to 22 volts. In some cases, for example, when anodizing rolled material or wire, alternating current is used. The use of a reduced current density makes it possible to obtain thin, transparent oxide films that are superior in transparency to films of similar thickness obtained at standard current densities.
Process duration
The duration of the anodizing process depends on the required film thicknesses, as well as the current density used. For pure aluminum this ratio can be proposed as:
Film thickness, microns. = (Current density, a/dm2 X Time, min.)/3
The ratio is approximate, since the duration of the process may depend on the type of alloy and processing mode.
The working process
The technological process of anodizing differs from the processes of applying galvanic coatings primarily in that the dissipative ability of anodizing electrolytes is significantly higher than that of electrolytes used in the processes of chrome plating, copper plating, galvanizing or nickel plating of metal. Effective dispersive ability with active mixing makes it possible to obtain films of uniform thickness over the entire surface of the products, including the internal surfaces of holes and grooves.
Otherwise, the technological process of anodizing is similar to the processes of electrochemical coating - products are immersed in a preheated electrolyte on hangers or clamps, the parts do not come into contact with each other, the distance to the cathode must be at least 15 cm (for larger products, the values are higher). Then the solution is stirred and current is applied. Under normal conditions, the cathode area should be equal to the anode area, and the cathode cross-section should be sufficient to ensure the required current density.
At the end of the process, stop the current supply and immediately remove the products from the galvanic bath. The products are washed in running water and dried.
Preparatory process
To obtain a smooth surface, the workpiece must be polished at the preparation stage. Using a felt or other polishing wheel, scratches are removed and large pores are tightened. The absence of micro-irregularities reduces the likelihood of burnouts. The anodic film is not able to hide external defects.
Before anodizing aluminum, it is necessary to determine the dimensions of the parts to be processed. The resulting layer is 50 microns thick, so it will be impossible to screw a nut onto the treated thread. If the parts are connected using a fit, then do not forget that after anodizing the parts cannot be ground.
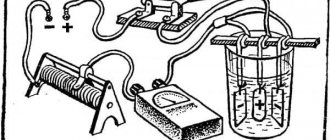
Carrying out anodizing at home
To carry out the process, containers are needed. Containers for anodizing must correspond to the dimensions of the parts, and be slightly larger. Therefore, they usually use several baths. The material of the containers is aluminum. But if the products are small, then plastic containers are suitable. Only aluminum sheets need to be laid on the bottom and along the walls. This is necessary to create a current of uniform density throughout the entire volume.
The electrolyte needs to be insulated from external heat. When heating it will have to be changed. To prevent heating, the outside of the container is covered with a layer of thermal insulation. It can be covered with polystyrene foam up to 50 mm thick or, placed in a box, the free space can be filled with polyurethane foam.
A sulfuric acid solution is prepared by diluting the electrolyte for car batteries with distilled water in proportions of one to one. By purchasing a canister with a capacity of 5 liters, you can get 10 liters of solution.
Mixing, when water is added to the acid, is accompanied by abundant heat, and it literally boils and splashes. Therefore, for safety reasons, sulfuric acid is poured into a container of water.
Before anodizing aluminum begins, it is subjected to chemical preparation. Chemical preparation is a degreasing process. In industrial conditions, treatment is carried out with caustic soda or potassium. But at home it is better to use laundry soap. A toothbrush and soap solution can easily remove dirt from the surface. After that, the workpieces are first washed with warm water, and then with cold water.
An alternative to laundry soap is washing powder. After dissolving it in a closed plastic container and placing the parts to be processed there, shake vigorously. Then the parts are washed and dried with a stream of hot air. The active oxygen contained in the washing powder protects fat-free products, even if handled with bare hands.
Anodizing in chromic acid
Chromic acid is used if it is necessary to anodize critical aluminum parts and assemblies with thin walls or with high processing precision. The dissolution of aluminum in chromic acid is lower than in sulfuric acid, the reduction in the fatigue strength of the metal is lower - the film turns out to be thin, opaque gray in color. The maximum thickness of the oxide film reaches 10 microns, the standard thickness is from 2.5 to 5 microns.
The concentration of chromic anhydride CrO3 is taken in the range from 2 to 15% (by weight). In most cases, the regime temperature is set within the range of 25-400C; active stirring of the electrolyte solution is not required. When anodizing in a 10% solution of chromic acid, the process temperature is raised to 540C at a voltage of 30 volts to ensure a current density of 1.2 A/dm2. For alloys containing copper or zinc, the voltage is set within 15-20 volts at the same current density. When anodizing in an electrolyte of low concentration 3-5% (by mass), a special voltage supply mode is used and the process proceeds in cycles. This mode is used to detect defects in the surface of a product or when forming a sublayer for painting.
What is anodizing
The anodizing process is an electrolytic chemical reaction of a metal with an oxidizing agent. A thin layer of oxide is applied to a metal surface, which acts as an anode during the reaction. Due to polarization in an electrolytic conducting medium, both pure metals and various alloys can be coated with a thin oxide film. The oxide layer effectively protects against corrosion and fading when exposed to direct sunlight. The most in demand in industry are anodized alloys of aluminum and magnesium.
The ultimate goal of anodizing is to create a so-called AOP - anodic oxide film - on the surface of an aluminum sheet. It performs two main functions:
- Protection from external influences;
- Decoration.
In the second case, dyes of various colors with a strictly defined chemical composition are added to the conducting medium.
Engineers from the UK were the first to introduce industrial anodization of aluminum into production. The light and durable metal created in this way began to be used in the aviation industry. Later, a standard for metal anodization appeared, which is successfully used in modern aircraft construction. It has the nomenclature marking DEF STAN 03-24/3.
The coating consists of two components:
- organic;
- anode-chromium.
Paint applied in accordance with the standard is very resistant to abrasion and other mechanical damage.
Anodization in oxalic acid
In a solution of oxalic acid, yellow films with high wear resistance are obtained. This method is one of the first open methods for obtaining a colored coating. The wear resistance of the coating during abrasion is two times higher than when anodizing in sulfuric acid. In the process of anodization in oxalic acid, along with direct current with a voltage of 30-60 volts, alternating current modes are used. To obtain a uniform yellow or bronze tint, the solution is vigorously mixed. Otherwise, this process is no different from anodization in sulfuric acid. Various metals can be used as cathodes - iron, lead, stainless steel.
The purpose of anodizing aluminum and its further use
Anodizing aluminum profiles and other parts makes a lot of sense. It is important that all the characteristics of the metal remain unchanged, but the surface of the product itself acquires additional qualities:
- A mechanically strong oxide layer is formed over the entire surface, which does not allow the metal to deteriorate under the influence of moisture and oxygen.
- Minor damage in the form of pinhole defects or minor scratches are hidden under the layer, and the metal becomes more uniform.
- When applying paint and varnish coatings, the latter are distributed more evenly and adhere well to aluminum.
- Parts made of anodized aluminum acquire a presentable appearance; they look advantageous on various mechanisms.
- During the anodizing process, you can give aluminum a completely different shade, for example, silver or gild it, or give it a pearly sheen.
Processed aluminum spare parts can be further used in the production of various components, machine mechanisms, and frames.
Other anodizing solutions
In some cases, electrolytes are used in which the aluminum oxide film does not dissolve - so-called barrier-type electrolytes . Using anodizing solutions containing boric acid, ammonium tartrate, and ammonium borate, coatings are obtained on parts used in electrical appliances (electrolytic capacitors). For example, when treated in a solution with ammonium borate, films are obtained with a breakdown voltage of 550 volts. Also, these types of electrolytes are used in anodizing aluminum deposited in a vacuum.
Aluminum parts, the processing of which involves the application of galvanic coating after anodizing, are processed in a solution containing 25-30% phosphoric acid. The resulting films have a thickness of up to 6 microns, which is due to the high solubility of aluminum in phosphoric acid. The process is carried out at workshop temperature, current density 10-20 A/mm2 and voltage 30-60 volts for 10-15 minutes.
Solid films of golden, brown or black colors are obtained by using a solution containing 40-100 g/l sulfosalicylic acid and 30-60 g/l sulfuric acid at a temperature of 300C, a current density of 2.5-3.5 a/dm2 and a voltage of up to 80 volts.
Electrolyte preparation
Acid solutions are considered unsafe reagents, so to anodize aluminum at home, they resort to a different type of solution. To prepare it, use salt and soda, which you always have on hand.
To prepare the electrolyte, take two plastic containers. They are filled with salt and soda compositions, observing the proportion: per serving of salt or soda 9 servings of distilled water.
Anodizing at home
After dissolving the components, the solution is kept to allow undissolved particles to settle to the bottom. When pouring into a container for anodizing, it must be strained.
Removing anodic coatings
Poor-quality anodic coating can only be removed from the entire surface of the product; partial restoration of the film is impossible in most cases. The coating is usually removed in solutions containing caustic alkalis. The process takes place under strict control of the main modes, since such solutions have a high degree of impact on the base metal. The classic solution that has the least impact on the aluminum surface is one containing 35 ml/l phosphoric acid and 20 g/ml chromic acid. Treatment takes place within 1-10 minutes, depending on the thickness of the film at a temperature of 95-1000C. to remove hard anodic coatings, use the specified solution with a concentration twice as high, while the surface of aluminum alloys containing copper can be painted gray or black.
Re-processing of products after removing the anodic film is possible after assessing the condition of the surface of the product; if the surface cleanliness is sufficient for coating and polishing is not required, you can begin the process immediately.
It should be noted that when processing parts for which it is necessary to strictly adhere to the original dimensions, repeated anodization will be required with the application of a film of greater thickness than it was originally. This is because when the coating is removed and reapplied, losses can range from half to two-thirds of the original film thickness.
You may be interested in the following articles:
|
Aluminum anodizing methods
Warm anodizing
This technology is considered relatively simple. You can repeat it yourself. The process is carried out at room temperature. With simple manipulations you can obtain a beautiful colored coating using organic dyes. If you put in some effort, you can get several colors on the same part.
It is worth remembering the Soviet weapons - RPO-2, RPS-3, RPO-3. These guns were green, a color that is the result of anodizing the aluminum. The dye used was brilliant green, which is sold in every pharmacy.
The technology has advantages, but there are also disadvantages. So, anodized aluminum treated in this way does not have really high corrosion protection. Corrosion occurs in sea water, as well as in places of contact with aggressive metals. Processing metal in this way also does not provide powerful mechanical protection - the surface is easily scratched by an ordinary needle. If the technology is broken, then the coating is completely erased by hand.
This coating serves as the basis for painting. It's hard to imagine such high adhesion. If, after anodizing an aluminum profile, you paint it with epoxy paint, you will get a very reliable coating and aesthetics. Epoxy paint will stay on the surface for a very long time.
Warm anodizing is very simple. The first step is to degrease the parts and secure them in the suspension. Anodize until milky and wash the part with cold water. Paint in a hot dye solution and fix the painted surface for an hour.
Cold technology
This method is performed at low temperatures – from -10° to +10°. The method was invented for several reasons: high quality, strength, hardness of the anode layer, as well as low surface dissolution rate and large layer thickness. Usually, at home, anodizing aluminum alloys is carried out in this way.
The layer on the metal side grows, and on the outside it dissolves. The speed is the same as for warm anodizing. However, cold technology can demonstrate low dissolution rates of the outer film. Because of this, a thick layer is formed. With the warm method, the outer layer dissolves as quickly as the inner one grows - it is much more difficult to obtain a solid film.
This technology requires good cooling of the parts - this is the only way to get a high-quality result. The coating will be hard and wear-resistant. So, salty sea water can no longer harm an underwater gun that is anodized in this way.
The only disadvantage of the procedure is the inability to use organic dyes. Dyeing is a natural process, and the color depends on the composition of the material being processed. The shades change during the process - from green to dark, often this technology produces a black color.
First, the part is degreased and secured in a special hanger. The metal is then anodized until a dense layer is obtained. Next, wash in hot or cold water. At the end, the layer is fixed by boiling in distilled water.
Hard anodizing technology
Hard anodizing of aluminum also produces a hard and durable film. This technology is widely used in industry. The peculiarity of this method is that the process involves not one, but several electrolytes. Thus, not only sulfuric acid is used, but also boric, tartaric, acetic or oxalic acid. The current density slowly increases and, due to changes in the structure, a film of increased strength grows on the surface.
Electrical equipment, light, lighting
0 votes
+
Vote for!
—
Vote against!
Every metal needs protection from rust and corrosion, including aluminum, which is very often used by ordinary people at home. If you create a dense and thick oxide film on the surface of aluminum, this will be quite enough to inhibit further corrosion, which is obtained in the process of anodizing aluminum. The most mechanically strong and resistant films are obtained by low-temperature thin-layer anodization of aluminum, which is what you will do.
Security questions
Carrying out high-quality anodizing at home is not difficult. It is safer and more convenient to do this work on the street or balcony. During the process, you will encounter several health hazards.
Acid is a very caustic thing. Although it is in a highly diluted form and causes only mild itching when it comes into contact with the skin, if it gets into the eyes it can cause serious injuries! Therefore, it is advisable to wear safety glasses when anodizing and always have a bucket of water or a weak soda solution on hand.
During the anodizing procedure, oxygen is released at the anode and hydrogen at the cathode. After mixing these gases, they form the well-known explosive gas, which, in principle, is the same as dynamite. Therefore, when anodizing indoors, you can die from the first spark.
Preparatory work
Remember that parts become larger after anodizing. The thickness of the protective anodic layer is usually 0.05 millimeters. For example, threads that were previously tightly tightened will no longer tighten at all after the anodizing process, since in this case the bolt in the nut will become 0.2 millimeters tighter. And it is almost impossible to polish anodized.
It is useful to polish the products to a mirror shine on a polishing wheel. Thus, the aesthetics of the part will greatly benefit and the likelihood of “burnout” during anodizing will be reduced. By the way, the anodic layer does not mask surface defects - they will be noticeable on the processed product.
Before galvanizing, aluminum must be thoroughly degreased. You should not keep the metal in hot caustic sodium or potassium, as is recommended in factory technologies, because the cleanliness of the surface noticeably deteriorates. It is better to use a bar of laundry soap and a toothbrush, because you will be working with small parts. First rinse the product in warm water, then in cold water.
Washing powder works very effectively: it needs to be dissolved in hot water in a plastic container. Then you should pour the products there and shake the vessel well. After washing, dry the parts thoroughly with hot air. Don’t worry about small traces of fat: after degreasing, you can pick up the product because the layer of fat from your fingers is instantly oxidized by oxygen.
Electrolyte production
The electrolyte for anodizing at home is a solution of sulfuric acid in distilled water. You can also use ordinary tap water, but if you can take distilled water, it is better to choose it, since in the first case the uniformity of the process—the distribution of current density on the surface of the part—is slightly deteriorated.
It’s stupid to make sulfuric acid yourself, but distilled water is very easy! If there is no snow or rain outside, then there will always be ice in the freezer. You can get distilled water and sulfuric acid at your local auto parts store, as these ingredients are used to service car batteries.
However, the acid is sold there in a diluted form to a density of 1.27 grams per cubic centimeter under the name “Electrolyte for a lead battery.” You need to mix this electrolyte with distilled water in a 1:1 ratio.
If you take a standard 5 liter canister of electrolyte and the same amount of water, you will end up with 10 liters of anodizing solution. This is enough for small parts, but for large parts it is worth doubling this amount.
Remember that mixing acid with water will generate a lot of heat. If you pour water into acid, it will instantly boil, splashing in your face! That is why it is recommended to pour the electrolyte into a container of water in a thin stream, stirring constantly with a glass rod. And it's better to wear safety glasses! If acid gets on clothing or skin, rinse it off immediately with a stream of water and rinse with a soda solution.
Processing modes
The temperature of the metal anodizing process is -10 - +10 degrees Celsius. A growing layer below -10 is quite good, but the voltage supplied by the power supply is not enough to maintain the required current. Above +10 degrees, although a protective film will form, it will be soft and colorless.
However, it is recommended to stop the anodizing process already at 5 degrees above zero. But the thing is, in the corner of the bath and on the surface of the part there is a different temperature, and during anodization a lot of energy is released in the form of heat.
But if forced mixing of the electrolyte is not ensured, you cannot trust the thermometer! However, you should constantly stir the electrolyte, with a spoon, with air, or with a pump, this is necessary to equalize the temperature on the surface of the aluminum product. Otherwise, areas of local overheating will form on the part, followed by breakdowns and ripping of the part.
The anode current density should be in the range of 1.6 - 4 Amperes per square decimeter. Within such limits, a beautiful, colored and dense protective anode layer will grow. It is best to maintain a current density of 2 to 2.2 Amperes/dm2. At a lower current, the coating will grow slowly and not thick. At a current greater than 4 Amperes/dm2, an electrical breakdown may occur and the product will quickly become etched.
The cathode current density must be low. The lower this indicator, the better, because this ensures a uniform and soft distribution of current density over the surface of the workpiece, especially if it is large. Therefore, remember that the area of the lead cathode should be twice the area of the part (anode).
The process of anodizing an aluminum profile does not specify the anode-cathode voltage. However, if your circuit has non-zero resistance, then you need a decent voltage power supply. Moreover, it is advisable that you use a power supply with multiple output voltages. And that's why.
The protective layer that grows on the product is a dielectric. As it increases, its electrical resistance constantly increases. To maintain the required current density, the current must be adjusted several times throughout the process using a variable resistor.
However, the voltage may not be enough when the anode layer becomes thick enough. In this case, you need to add voltage. Therefore, the power supply must provide at least two output voltages.
Anodizing bath
Before work, it is necessary to prepare equipment for anodizing. Usually several baths are required: for processing small parts, short and long products. They must be made of aluminum. Polyethylene is also a suitable option. As a small container, you can use a food container or a long plastic flower pot.
It is advisable to cover the bottom and walls of the plastic bath with aluminum sheets. You can cut a pattern from a sheet of aluminum and bend an improvised “container”. The point of this is to ensure uniform current density on all sides of the product.
The bath must have good thermal insulation of the body, otherwise the electrolyte in it will heat up too quickly and will have to be changed more often. The simplest solution would be to cover the bathtub with a thick layer of polystyrene foam - 2-4 centimeters. You can also secure the bathtub inside the box and fill the gap with construction foam.
After this, a lead cathode should be made for the bath. It can be made from sheet lead by removing the latter from thick electrical cables. Let us recall that the cathode area should be twice the surface area of the workpiece. This does not take into account the surface of the cathode, which is leaning against the wall. The cathode plate must have holes for gas outlet.
You can build a cathode from several pieces of lead if you don't have one. It is recommended to solder the pieces with a powerful soldering iron, with a thick seam along the joints. Try to ensure that the cathode follows the configuration of the surface of the part facing it. Remove the contact from the bath using a strip of the same material. Although it is also common to use thick copper wire in insulation. Insulate the soldering area with silicone sealant.
Anodizing process
So, you have poured electrolyte into a plastic bath, and at the output there is a power supply with current. To regulate the current in the circuit when anodizing titanium or aluminum, connect a wirewound variable resistor. There are 2 items in the container: a lead cathode in the form of a plate and an anode - the product being processed. When current is applied to them, oxygen is released and the anodic protective layer begins to grow.
When creating high-quality electrical contact between the lead and the part, you will observe oxygen microbubbles that slowly rise from the entire surface of the product. Their diameter is extremely small, their flow resembles wisps of smoke. The duration of the process should be controlled visually - by the color of the part.
For small parts it takes 20-30 minutes, for large items - an hour and a half. After the part is completely covered with a gray-blue coating, it should be removed from the bathroom, washed under running cold water and wiped with a cotton swab dipped in a strong manganese solution to remove reaction by-products. The surface should be shiny, light gray, smooth.
After the anodizing process at home, some products acquire a dark matte shade, it all depends on the anodizing mode. To paint anodized products, immerse them in a solution of aniline dye, which is heated to 50-60 degrees Celsius. Before work, filter the solution, because small grains of undissolved dye can form stains on the metal surface. The color intensity usually lasts no more than 15-20 minutes.
After the part has acquired a beautiful shade and a hard, not loose protective layer, it is necessary to fix it. The fact is that this coating at the micro level has a porous structure that is permeable to air and water. This metal layer protects well from mechanical damage, but is weak against chemical damage.
There are several methods that help close micropores. The simplest is to boil the parts in a pan in water for half an hour after anodizing. It is better to use distilled water. Also, the parts can be kept in a steam bath, also for half an hour.
You already know that there are several technologies for anodizing aluminum and parts made from it. They differ in the working process conditions, and more specifically, in the temperature of the electrolyte, which is the main factor that affects the quality of the anodic protective layer. At home, it is preferable to choose the option of cold anodizing, because in this case the coating is of higher quality and thicker, and the part acquires a beautiful shade and shine.
Warm anodizing
The warm anodizing process is carried out at an ambient temperature of 15-20 °C. Parts processed in this way have two negative features:
- Not very high anti-corrosion resistance. When in contact with a chemically aggressive environment or metal, the anodized layer is exposed to oxygen.
- Low degree of protection against mechanical influences. It is quite possible to cause mechanical damage to the anodized layer with a sharp tip.
The warm anodizing process consists of six stages:
- cleaning the surface of the part from grease.
- fastening on a suspension.
- anodizing until a light milky color appears.
- rinsing with cold water.
- dyeing with a hot solution of aniline dye.
- allowing the anodized metal to sit for 30 minutes after painting.
The layers of film produced by warm anodizing are exceptionally beautiful. This type of aluminum is best used in structures that are not exposed to harsh external influences. In addition, the anodized layer is an excellent base for repainting due to its superior dye adhesion. The applied paint will last for a very long time.
Application of anodized aluminum
There are many areas of use to achieve completely different goals. Now let's look at them:
- Base for painting. The protected coating is able to retain a layer of paint for a long time. To do this, the organic coating is combined with a chrome anode. Even if the paint layer is damaged, it is easy to restore, and the product itself is not in danger of corrosion, etc. This technology is effective when applying organic paints.
- Corrosion protection. This protection is able to cope with the effects of even salt water.
- In design. Using special dyes, aluminum can be given completely different colors. Thanks to this, products can be given a beautiful appearance.
- Clean hands. Aluminum is often used to create railings, handles, handrails, etc. If it is without an anodic coating, then marks may remain on your hands. To eliminate this, all these parts are anodized, which allows you to keep your hands clean. To achieve such results, the pores of the anodic coating are filled.
- Reflection in projectors. Sulfate anodizing technology is used to protect floodlight reflectors. This reflection will last for years. And if you need to clean its surface, then there is no problem.
- In thermal reflectors. Anodized aluminum is used in heating reflectors. The surface is easy to clean. Can be used in rooms with high humidity. The coating thickness is 1 micron.
- Effectively combats wear and friction. Due to the harder coating, wear is significantly reduced. In this case, the anodic coating can reach up to 60 microns.
- Electrical insulator. In some types of transformers today it is customary to use aluminum tape, which must be anodized. This coating perfectly resists the effects of thermal energy.