Various European standards include more than 150 wrought aluminum alloys. However, only 17 of them are used in the construction of buildings according to Eurocode 9 [1]. An important condition for choosing these alloys is their level of corrosion resistance under various operating conditions of buildings with a minimum of maintenance. The following overview of the main types of aluminum corrosion provides an understanding of why some aluminum alloys can be used in construction and others cannot.
See more Construction aluminum alloys
Natural oxide coating
The natural surface of aluminum, which is created during the manufacturing of an aluminum product, such as extrusion, rolling or casting, has high resistance to corrosion in most types of environments. This occurs because a fresh aluminum surface spontaneously and instantly forms a thin but very effective oxide layer that prevents further oxidation of the metal.
This oxide film is impermeable and, unlike the oxide films of other metals, such as iron, is very firmly “attached” to the base metal. In case of any mechanical damage, this film is instantly restored and healed.
The natural oxide layer is the main reason for aluminum's good resistance to corrosion. This coating is resistant in environments with acidity - pH value - from 4 to 9.
How to protect metal from damage
The work uses several types of protective equipment, which include:
Use of special paints and varnishes
As with outdoor rust control, it is very important to prevent the corrosive environment from coming into contact with the metal. In this case, LMBs are perfect.
There are several types of materials that can be freely used in dyeing.
These include:
- Bitumen-based paints.
- Compositions with a phenol-formaldehyde base.
- Ethanol paints and varnishes.
Substances with an epoxy and coal tar base perform well. The main requirement is that they contain as few solvents as possible.
The main advantage of using such a product is its ease of application.
The paint is applied to the surface, the protected areas immediately become clearly visible.
For additional enhancement, protecting the effect from an aggressive environment, you can also use various oxides, including mercury and copper. In this case, the structure will not become overgrown with marine life.
In order for the application of paintwork materials to give the best results, the surface of the metal structure will need to be phosphated. Only after this is staining allowed.
It is also worth considering that it should be as thick as possible in order to last longer and maintain a noticeable effect.
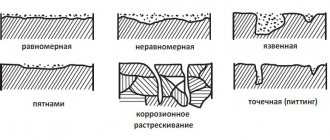
Three main types of aluminum corrosion
The most common types of aluminum corrosion are:
- galvanic (contact) corrosion;
- pitting (pitting) corrosion;
- crevice corrosion.
Stress corrosion, which leads to cracking, is a more specific type of corrosion. It occurs primarily in high-strength aluminum alloys, such as AlZnMg alloys, when they are subjected to prolonged tensile stress in the presence of a corrosive environment. This type of corrosion does not usually occur in 6xxx series alloys, i.e. AlMgSi alloys.
Chemical resistance of copper and its alloys.
The standard copper potential is +0.52/0.337V for the reduction of cuprous and divalent copper, respectively. Typically, during corrosion, copper goes into solution in its divalent form. The standard potential of copper in a solution of 3% sodium chloride is +0.05V, and in a solution of 1N hydrochloric acid is +0.15V. Therefore, copper under normal conditions does not displace hydrogen from solutions, i.e. cannot corrode with hydrogen depolarization. The ability to passivate copper is weakly expressed. Resistance to gas corrosion of copper increases when alloyed with beryllium, magnesium and aluminum.
Brass is an alloy of copper and zinc. The introduction of aluminum, manganese, and nickel into brass increases the resistance of the alloy to atmospheric corrosion, and silicon to sea water.
Copper is resistant:
- In saline solutions;
- In dilute non-oxidizing acids;
- In formaldehyde.
Copper is unstable:
- In solutions where it can form complexes (cyanides, ammonia);
- In solutions of oxidizing agents - nitric acid, hydrogen peroxide;
- In the presence of dissolved oxygen (especially when blowing it through the solution);
- In chromic acid;
- In formic acid;
- In sulfides, polysulfides, sulfur dioxide.
Galvanic corrosion of aluminum
Galvanic corrosion can occur when two different metals are in direct contact and an electrolytic bridge has formed between them. The less noble metal in this combination becomes the anode and corrodes. The more noble metal becomes the cathode and is protected from corrosion.
In most combinations with other metals, aluminum is the less noble metal. Aluminum is therefore at higher risk of galvanic corrosion than other building materials. However, this risk is less than is generally believed.
Necessary conditions: contact and moisture
Galvanic corrosion of aluminum occurs only when simultaneously:
- there is contact with a more noble metal (or other electrical conductor with a higher chemical potential than aluminum, such as graphite;
- between two metals there is an electrolyte with good conductivity, most often water with dissolved salts.
Galvanic corrosion does not occur in a dry air atmosphere, such as inside a normal living space. There is no big risk of galvanic corrosion and a clean rural atmosphere. However, the risk of galvanic corrosion must always be taken into account in atmospheres with high chloride content, for example in areas near seas and oceans.
Aluminum and galvanized steel
There may be problems with galvanic corrosion when pairing aluminum with galvanized steel. The zinc coating on galvanized steel will initially protect the aluminum from corrosion. However, this protection decreases when the surface of the steel begins to be exposed as the zinc is consumed. Hot-dip galvanizing of steel produces a thicker zinc coating than electrochemical galvanizing and provides longer-lasting protection for aluminum. Therefore, in aggressive atmospheres in contact with aluminum, only hot-dip galvanized steel is used.
Electrical insulation
Where different metals are used in contact, galvanic corrosion can be avoided by electrically isolating one metal from the other. An example of such a solution for a bolted connection between aluminum and steel sheet is shown in Figure 1. Electrolyte may occur between the bolt head and the surface of the aluminum, but the electrical insulating washer will not allow galvanic electric current to flow and corrosion will not occur. On the other hand, there is no possibility of moisture ingress in the contact between aluminum and steel sheets, electrolyte is not formed and corrosion does not occur.
Figure 1 – Electrical insulation of aluminum from steel
Break in the electrolytic circuit
In large structures, where the use of electrical insulation is difficult, an alternative solution is used - preventing an electrolytic bridge between the two metals. Surface painting is one way to do this. Most often, the best option is to paint the surface of the cathode, that is, a more noble metal.
Cathodic protection
Cathodic corrosion protection can be achieved in two ways. Most often, this is the installation of an anode made of a less noble metal in direct metal contact with aluminum. This less noble metal “sacrifices” itself, that is, it corrodes instead of aluminum. Therefore it is called a sacrificial anode.
For such a sacrificial anode to work, it must be in liquid contact with the aluminum surface being protected. To protect aluminum, zinc and magnesium are most often used as sacrificial anodes. An example of cathodic protection is shown in Figure 2.
Another way to obtain cathodic protection is to connect an aluminum object to the negative pole of the rectifier.
Figure 2 – Cathodic protection of an aluminum ship propeller
Chemical resistance of lead.
The standard lead potential is -0.126V. The corrosion resistance of lead is largely determined by the stability of its corrosion products.
Lead resistant:
- In sulfuric acid and sulfates;
- In phosphoric acid and phosphates;
- In hydrochloric acid up to 10%;
- In hard waters with calcium sulfate;
- In silicic acid;
- In industrial atmospheres with hydrogen sulfide, sulfur dioxide and sulfuric acid.
Lead is unstable:
- In nitric acid;
- In acetic acid;
- In alkalis;
- In sulfuric acid above 96% and oleum;
- In hot sulfuric acid up to 80%;
- In hydrochloric acid over 10%;
- In groundwater with organic acids;
- In underground waters saturated with carbon dioxide.
Pit corrosion of aluminum
For aluminum, pitting corrosion is the most common type of corrosion. It also occurs only in the presence of an electrolyte (water or moisture) that contains dissolved salts, usually chlorides.
This corrosion usually appears as very small pits that, when exposed to air, reach a maximum depth of a small fraction of the thickness of the metal. The depth of these pits can be greater in water and soil.
Prevention of pitting corrosion
Pitting corrosion is mainly an aesthetic issue because, in a practical sense, it never reduces the strength of aluminum products.
The manifestation of pitting corrosion, of course, is more serious on aluminum with a natural surface, that is, a surface without any protective treatment. Protective surface treatment of aluminum (anodizing, painting or other coating methods) successfully protects it from pitting corrosion.
To prevent pitting corrosion, cathodic protection is also used (see above).
Drainage design
It is very important to design aluminum profiles and other aluminum products so that they have the ability to drain precipitation and quickly dry the surface. Profiles that may be exposed to moisture should not have corners or pockets in which water accumulates. Each profile in which water can accumulate must have drainage holes (Figure 3).
Figure 3 – Structural drainage in aluminum profiles
Effective drainage (Figure 4) and ventilation of “wet” aluminum profiles significantly reduces the risk of pitting corrosion on them.
Figure 4 – Drainage holes in aluminum profile
Chemical resistance of titanium.
The standard potential of titanium is -1.63/-1.21V for the divalent and trivalent forms, respectively. Titanium is prone to passivation.
Titanium resistant:
- In oxidizing environments (including chromates, permanganates, hydrogen peroxide, oxygen, nitric acid);
- In the presence of chloride ions;
- In aqua regia;
- In iron (III) chloride up to 30% and up to 100o C;
- In copper (II) chloride up to 20% and up to 100o C;
- In mercury (II) chloride of all concentrations up to 100 ° C;
- In aluminum chloride up to 25% and up to 60o C;
- In sodium chloride of all concentrations up to 100o C;
- In sodium hypochlorite solution up to 100o C;
- In chlorine water;
- In gaseous chloride up to 75o C;
- In hydrochloric acid no more than 3% at 60o C;
- In hydrochloric acid no more than 0.5% at 100o C;
- In phosphoric acid up to 30 no higher than 35o C;
- In phosphoric acid up to 3% at 100o C;
- In a humid chlorine atmosphere (in the presence of above 0.005% moisture);
- In alkalis up to 20%;
- In many organic environments.
Titan is unstable:
- In hydrochloric acid above 3% at 60o C;
- In hydrochloric acid more than 0.5% at 100o C;
- The maximum dissolution of titanium in sulfuric acid is observed at 40% and 75%;
- In an atmosphere of absolutely dry chlorine;
- In alkalis above 20%.
Crevice corrosion of aluminum
The essence of crevice corrosion
Crevice corrosion can occur in narrow, fluid-filled crevices. The occurrence of such corrosion in aluminum profiles is unlikely. However, significant crevice corrosion can occur in the marine atmosphere or on the exterior surfaces of vehicle bodies. During transportation and storage of aluminum profiles, water can sometimes collect in the crevices between adjacent aluminum surfaces, causing surface corrosion in the form of “water spots” (Figure 4).
Figure 5 – Essence of crevice corrosion
The source of this water is rain or condensation. This water is literally sucked through a capillary mechanism into the space between two metal surfaces. Moisture condensation can occur when cold material is placed in a warm room. The difference between night and day temperatures can also cause condensation when aluminum is stored outside under a thick awning that prevents ventilation.
Chemical resistance of tin and its alloy with bismuth.
The standard potential of tin is -0.136V. Pure tin is compact at temperatures above +13o C (in the form of white tin). Below this temperature, especially at -48o C, tin actively transforms into the allotropic modification “gray tin”, which has a powdery structure. To eliminate this phenomenon, tin is doped, for example, with a small amount of bismuth (0.5-2%). Tin is weakly passivated.
Tin is stable:
- In natural waters;
- In solutions of neutral salts;
- In food environments;
- In dilute solutions of sulfuric and hydrochloric acids;
- In organic acids.